Chimeric antigen receptor (CAR) T cells have successfully induced remission in patients with relapsed or refractory B cell malignancies, but a substantial portion of patients eventually relapse. Although CAR expansion and persistence are considered important attributes for long-term benefit, systematic evaluation of contributing factors has been complicated by reliance on xenograft models. In a paper published in Science Translational Medicine, Yang et al. overcame this limitation by using transgenic TCR mice and uncovered some unexpected findings surrounding the biology of the dual antigen specificity (CAR and TCR) of CAR T cells and how it affects their antitumor response.
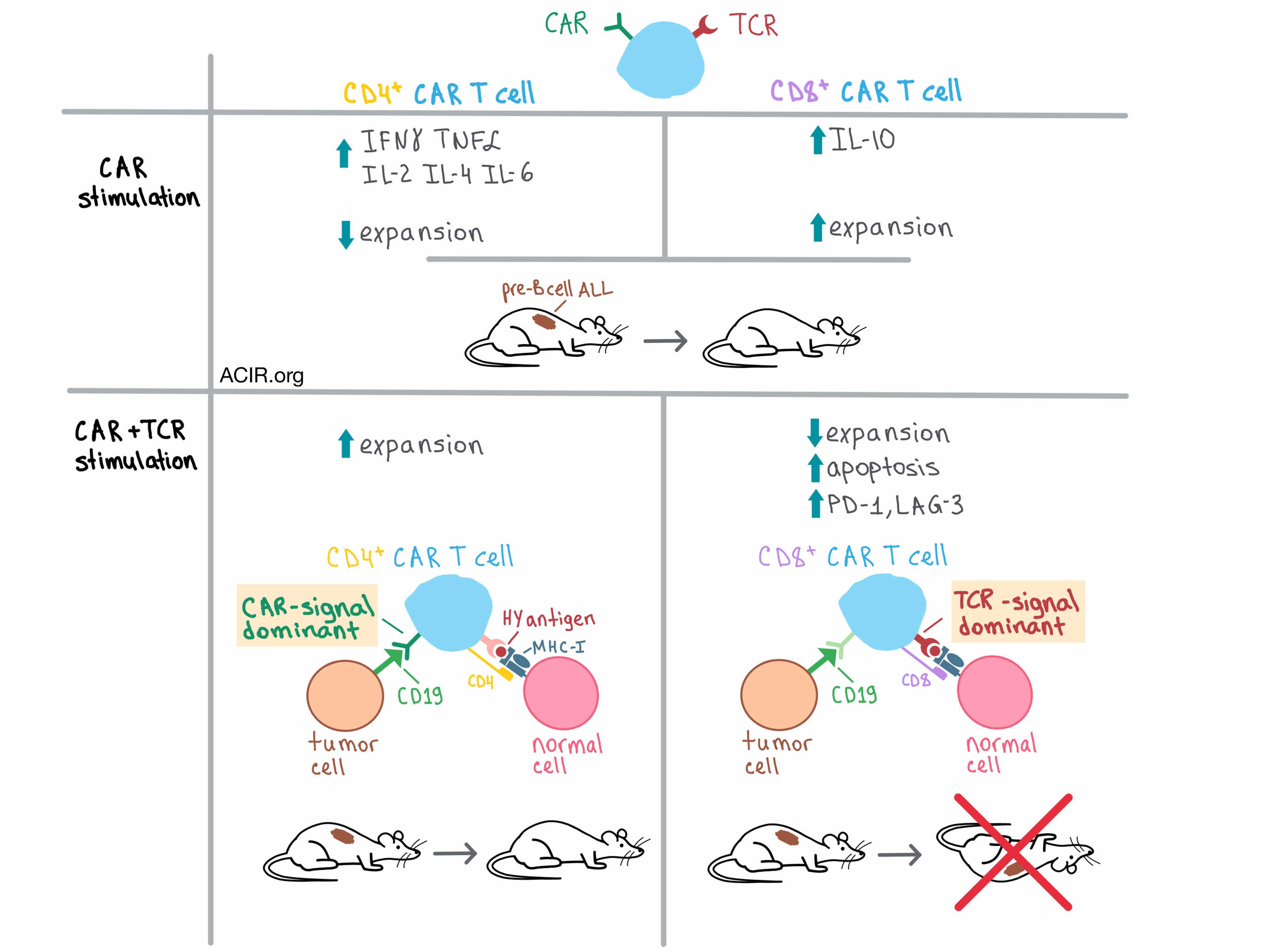
In the initial experiments, the team found that CD4+ and CD8+ T cells transduced with a murine CD19 CAR (CAR4 and CAR8 cells, respectively) were equivalently cytotoxic in vitro against syngeneic CD19+ pre-B cell acute lymphoblastic leukemia (ALL). A closer look, however, revealed important differences between the two CAR T cell types stimulated through the CAR. For example, CAR4 cells produced significantly more immunostimulatory cytokines, such as IFNγ, TNFα, IL-2, IL-4, and IL-6, than CAR8 cells, while CAR8 cells produced more immunosuppressive IL-10. On the other hand, the expansion of CAR4 cells was reduced in the presence of CAR8 cells, which were preferentially expanded.
To explore the effect of dual activation of CAR T cells on antitumor efficacy in vivo, Yang et al. utilized their CD19+ pre-B cell ALL model together with syngeneic CAR4 and CAR8 cells from transgenic mice with endogenous TCRs specific for a male minor histocompatibility antigen (HY). In a clever experiment, the team adoptively transferred HY-specific CAR4 or CAR8 T cells from female donors into ALL male (HY+) and female (HY-) mice, thus allowing the researchers to isolate the effects of CAR stimulation from the influence of TCR signaling. They found that CAR4 cells eliminated leukemia in both female and male mice and improved survival to >100 days. While CAR8 cells had a similar effect on female mice, they failed to clear leukemia and prolong survival in males relative to control mice. These results indicate that TCR signaling abrogates the ability of CAR8, but not CAR4 cells to eradicate leukemia, hinting at innate biological differences between the two types of CAR T cells.
In an attempt to figure out why CAR8 cells did not eliminate leukemia in HY+ mice, Yang et al. analyzed the adoptively transferred CAR T cells and found that the presence of the TCR antigen preferentially reduced the expansion of the CAR8 and not CAR4 cells, and that this was due to increased CAR8 apoptosis. Further analysis revealed that in female mice, both CAR4 and CAR8 cells had elevated PD-1 and LAG-3 exhaustion markers upon CAR stimulation. While the presence of TCR antigen in male mice did not further increase these markers on CAR4 cells, CAR8 cells became even more exhausted.
The team then created female/male hematopoietic chimeras via CD3-depleted bone marrow transplantation and found that even when the HY antigen was restricted to the hematopoietic tissues, CAR8 cells failed to eliminate leukemia, whereas CAR4 cells cleared the tumor and prolonged survival regardless of where HY was expressed. Furthermore, the hematopoietic restriction did not prevent CAR8 exhaustion. Similar results were observed in the OVA-specific OT1 transgenic mouse model, suggesting that these results apply to TCR-stimulated CD8+ CAR T cells in general. These experiments provide further evidence of an inherent, selective resistance of CAR4 cells to the negative effects of TCR stimulation. However, it is important to note that although CAR4 cells were successful in initial expansion and tumor clearance, they did eventually become exhausted and apoptotic upon chronic TCR antigen exposure, suggesting that long-term persistence of CAR4 cells and surveillance of leukemia may still be impaired.
In order to get to the root of the biological differences between CAR4 and CAR8 cells, Yang et al. examined the differential gene expression profiles of these cells after stimulating them with either CAR antigen, TCR antigen, or a combination of the two. Stimulation through either receptor alone generated distinct patterns in both CAR4 and CAR8 cells, but dual stimulation demonstrated that the TCR signal dominated in CAR8 cells, while the CAR signal dominated in CAR4 cells. In addition, CAR8 cells exhibited an increased expression of proapoptotic and inhibitory receptor genes after TCR stimulation, consistent with the phenotypic results.
Overall, these results raise awareness of the importance of TCR specificity and antigen presentation to CAR cells with endogenous TCRs, and indicate multiple pathways that could be targeted to improve the performance and persistence of CAR T cells and enhance the therapeutic potential of CAR T cell therapy.
by Anna Scherer




