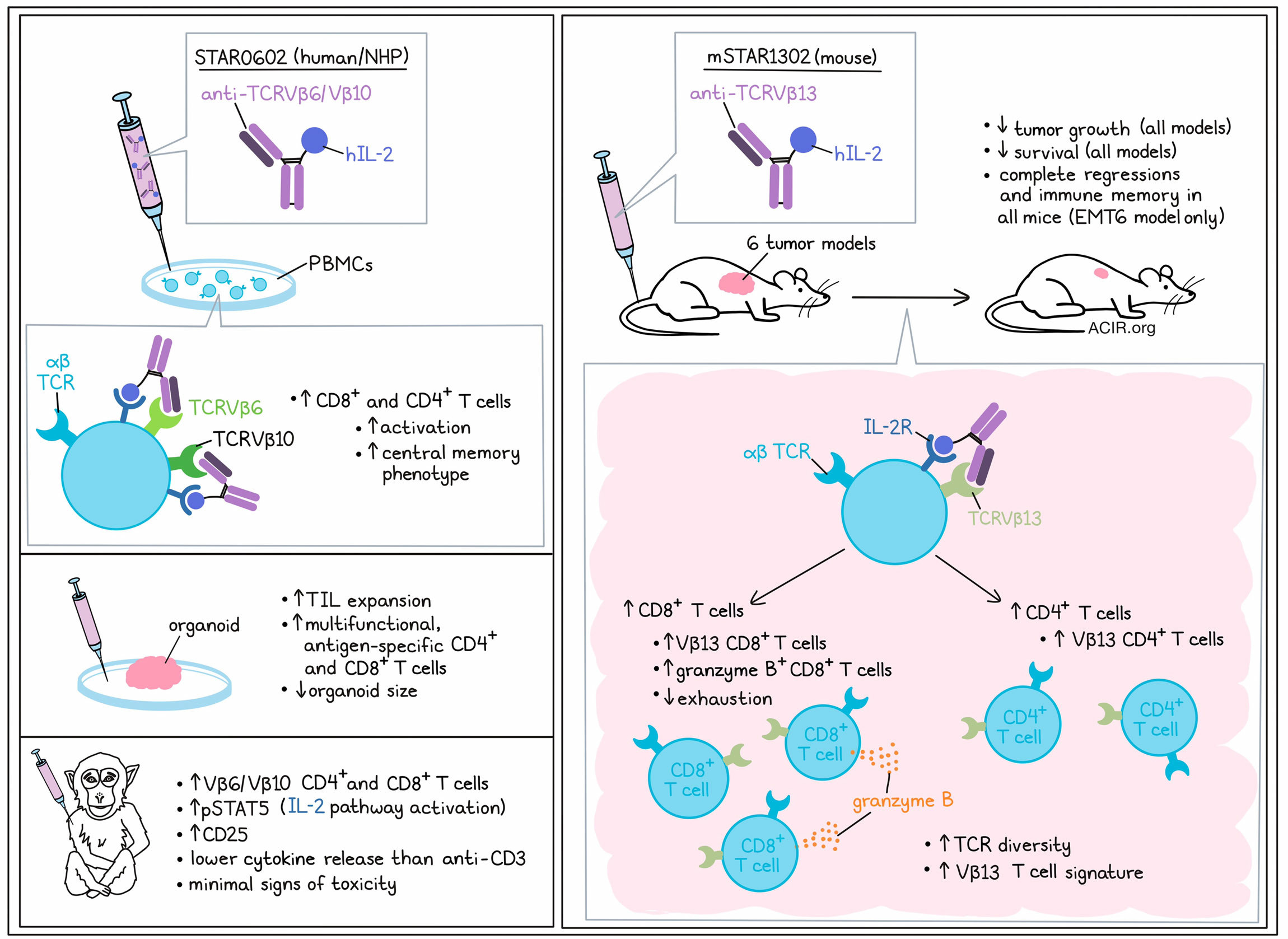
Stimulating αβ T cells through the T cell receptor (TCR) complex using CD3-agonistic antibodies as immunotherapy remains a challenge, as it activates all T cells, resulting in cytokine release syndrome and inefficient T cell activation. To overcome this issue, Hsu, Donahue, et al. designed and tested a bifunctional antibody fusion molecule fused to an IL-2 molecule to selectively activate and expand a commonly observed subset of tumor-infiltrating lymphocytes (TIL). Their preclinical data were recently published in Science Translational Medicine.
The researchers developed a fully human bifunctional antibody fusion molecule (STAR0602) comprising an antibody that binds germline Vβ6 TCRs (with some binding to the related Vβ10 TCRs) of human αβ T cells, fused with a native human IL-2, with the objective of achieving selective activation and expansion of CD8+ and CD4+ Vβ6/Vβ10 T cells by simultaneous engagement of TCR activation with IL-2R binding and activation in cis on the same cell. The Vβ6 TCR T cell subset was chosen because of its high prevalence relative to other Vβ subsets in TIL, and its commonality among different cancers.
In vitro, STAR0602 activated both CD4+ and CD8+ T cells, but increased the number of CD8+ T cells to a greater extent than CD4+ T cells. STAR0602 induced a central memory T cell phenotype (Tcm), possibly because it results in the co-clustering of TCRs with IL-2 receptors on the surface of targeted T cells. This was confirmed by assessing signaling pathways downstream of the TCR and IL-2R. In CD8+ T cells, pERK, pSLP76(Tyr145), and pSTAT5 were increased. Furthermore, in STAR0602-stimulated CD8+ T cells, there was an upregulation of CD25, intracellular granzyme B, and IFNγ, further confirming a Tcm phenotype, but with atypical expression of some effector markers.
For mouse experiments, a surrogate molecule was developed (mSTAR1302) that comprises the same human IL-2 antibody fusion design, but targets and expands the mouse Vβ13 CD4+ and CD8+ T cell subsets. Treating BALB/c mice with mSTAR1302 induced expansion of Vβ13 CD8+ and, to a lesser extent, CD4+ T cells in the blood, while it did not impact Tregs. In toxicity tests, no perivascular inflammation or liver enzyme changes were observed.
To determine the antitumor effects of mSTAR1302, the researchers made use of six syngeneic solid tumor models with varying degrees of T cell infiltration, tumor microenvironments, and responses to immune checkpoint blockade (ICB). In all six models, mSTAR1302 monotherapy induced tumor growth inhibition, and complete regression was observed in all mice in the EMT6 breast cancer model. Treatment improved survival in all models, and this improvement in survival was higher than that induced by anti-PD-1 monotherapy.
The researchers further investigated the mechanism of action of mSTAR1302 in the EMT6 model. In treated tumors, higher proportions of CD8+ T cells, Vβ13 CD8+ T cells, and granzyme B+CD8+ T cells were observed, which resulted in an increase in the CD8/Treg ratio. There were no changes in the NK or B cell populations, but there was an increase in total CD4+ T cells, Vβ13 CD4+ T cells, and the CD4/Treg ratio. Confirming the role of Vβ13 T cells for the efficacy of this treatment, selective depletion of this subset completely negated the antitumor effects of mSTAR1302. Furthermore, depletion of CD8+ T cells and, to a lesser extent, CD4+ T cells, reduced the antitumor activity of the treatment. Cured mice rechallenged with EMT6 were specifically protected against tumor growth, suggestive of the development of memory responses, which were CD8+ T cell-dependent.
To assess the immune mechanisms of treatment, TILs from the EMT6 model were subjected to transcriptome analysis. Treatment induced an increase in CD8+ naive, effector, and effector memory (Tem) T cells, as well as CD4+ T helper cells, while there was a decrease in the number of Tregs and CD8+ exhausted T cells. Most Vβ13 T cells were clustered into the CD8+ Tem cluster. Single-cell RNA sequencing data also showed increases in CD8/Treg, CD8/CD4, and non-exhausted/exhausted CD8+ T cell ratios. Comparing Vβ13 T cells in terms of differentially expressed genes (DEGs), treatment prompted a distinct gene signature, which was most pronounced in CD8+ Tem cells, and was characterized by an upregulation of T cell effector, memory, and cytotoxic genes, and downregulation of T cell exhaustion genes, TCR signaling repressors, and checkpoint molecules.
TCR sequencing revealed an increase in the frequency of unique CDR3s with smaller clonal sizes in the expanded Vβ13 T cell subset, while non-Vβ13 T cells maintained a low TCR diversity, with only a single CDR3 clonal expansion. Various analyses confirmed the increase in TCR diversity in the Vβ13 T cell population in response to treatment. To further assess the functional relevance of this increase in diversity, ex vivo antigen recall assays were performed on spleen-isolated T cells obtained from mice with EMT6 tumors treated with mSTAR1302. This resulted in robust T cell responses in Vβ13 CD8+ T cells challenged with cognate EMT6, but not B16F10 or CT26, tumor lysates.
After confirming the reactivity of STAR0602 with cynomolgus Vβ6 and IL-2 receptors, STAR0602 was then assessed in non-human primates. A single dose was rapidly cleared, but it induced a sustained expansion of the Vβ6/Vβ10 CD8+ and CD4+ T cell populations in the periphery. After treatment, there was an increase in pSTAT5, indicative of IL-2R pathway activation, as well as increases in serum CD25 concentrations. Limited cytokine release was observed, and peak concentrations were 50-90% lower than those induced by agonistic CD3 antibody treatment. Markers associated with IL-2 toxicity were minimally increased, and there were no signs of hepatoxicity, other serious toxicities, body weight loss, or deaths.
Finally, the researchers assessed the in vitro activity of STAR0602 in human rectal tumor organoids. STAR0602 induced expansion of TIL in all tumor models. In three out of four models, of which two were refractory to pembrolizumab, treatment also reduced organoid size. Furthermore, STAR0602 could expand HPV-16-specific CD4+ and CD8+ multifunctional T cells when stimulated with HPV-16 peptides in vitro.
In summary, these data suggest that STAR0602 can effectively target and activate a common subset of Vβ T cells to promote antitumor responses in human in vitro and murine in vivo models, and was found to be safe in non-human primates. Given its effects in models refractory to ICB, this novel mechanism of action may be an interesting approach for immunologically cold and therapy-resistant tumors. It is currently being evaluated in early-phase clinical trials.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, co-lead authors Andrew Bayliffe and Jeffrey Schlom answered our questions.

What was the most surprising finding of this study for you?
Out of the thousands of scientific publications covering T cell therapeutics for cancer over the last decade, selective expansion of T cells expressing germline-encoded TCR β-chain alleles is a completely unique mechanism. More importantly, we were pleasantly surprised to find that our STAR0602 molecule promoted a strong memory phenotypes in various models, which we immediately recognized as being potentially clinically useful.
What is the outlook?
We believe STAR0602 could be game-changing for patients who fail to respond to checkpoint inhibitors, because it promotes antitumor activity via a very different mechanism, characterized by expansion of a new subset of memory Vβ T cells that reinvigorate the T cell response to tumors. To this end, STAR0602 is being tested in a Phase 1/2 clinical trial in patients with advanced metastatic solid tumors. In the meantime, we will also be exploring how to combine STAR0602 with other cancer therapies to further enhance treatment options for patients.
What was the coolest thing you’ve learned (about) recently outside of work?
We both recently read about the discovery of a supermassive black hole by the Webb Telescope that is as large as some galaxies, which, by analogy, reminds us that there is also still much to learn about the “dark matter” of the immune system!




