In cancer immunotherapy, reversing or staving off T cell exhaustion is highly useful, and while current checkpoint blockades show substantial efficacy, they don’t appear to rewire the epigenetics that drive exhaustion in the first place. Investigating the epigenetic programming of exhaustion and potential ways to mitigate it, Beltra et al. identified Stat5 as an antagonist to Tox-mediated exhaustion programming, and interrogated ways to utilize this pathway to improve immunity.
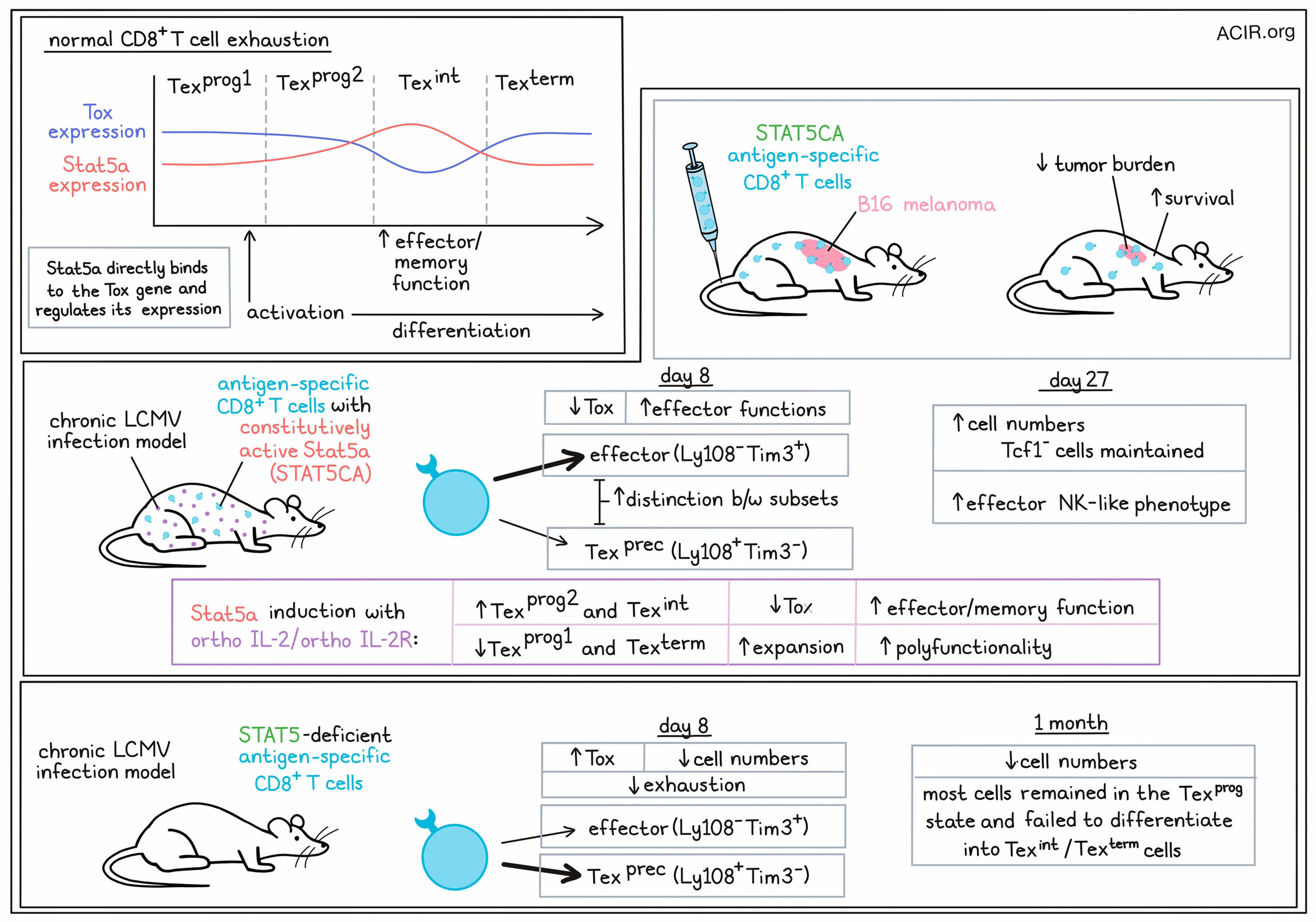
T cell exhaustion occurs in a distinct lineage of T cells (Tex), which progress from progenitor (Texprog) to intermediate (Texint) to terminally (Texterm) exhausted states. Based on previous research showing that expression of the transcription factor Tox, which mediates exhaustion programming, is lower and some effector functions are higher in Texint cells, Beltra et al. investigated whether Tox may have an antagonist in this setting. Looking at several sets of gene expression data, the researchers identified the Stat5a transcription factor as being positively correlated with effector and memory T cell functions, and inversely correlated with Tox and exhaustion-related genes.
To better understand the role of Stat5a in T cell exhaustion, the researchers evaluated viral antigen-specific CD8+ T cells transduced with constitutively active Stat5a (STAT5CA) in chronic LCMV-infected mice. At day 8, the T cells had differentiated into either effector-like (Ly108-Tim3+) T cells or Tex precursors, (Ly108+Tim3-). Compared to control cells, a higher portion of STAT5CA cells differentiated into effector-like cells, forming fewer Tex precursors. STAT5CA cells also showed lower levels of Tox per cell and had increased expression of effector-related molecules.
Next, the researchers evaluated the role of Stat5a through genetic deletion of Stat5a-b. In mice treated with these cells, then infected with chronic LCMV, the researchers found that a higher proportion of Stat5a-b-deleted cells were Tex precursor cells, with very few becoming effector-like cells. Further, these cells expressed higher levels of Tox, and were significantly reduced in number compared to control cells, suggesting that Stat5 plays a role in effector differentiation.
Returning to STAT5CA model, the researchers performed ATACseq on cells taken at day 8 post-infection, and found that compared to control cells, the effector-like and Tex precursor-like cell subsets in STAT5CA showed unique profiles, and were more distinct from one another, suggesting that constitutive expression of Stat5a drives diversion. Analysis of differentially accessible peaks (DAPs) showed that the majority of changes in STAT5CA cells reflected reduced exhaustion and a shift towards a more effector-like state. Further, analysis of open chromatin regions showed that a large portion of genes where accessibility varied between STAT5CA cells and control cells contained one or more Stat5 binding sites. The Tox locus itself contained some of the highest numbers of direct Stat5-binding sites, and both Tox and Tox-dependent regions were less accessible.
Looking at the longer-term impacts of constitutive Stat5a expression, the researchers evaluated cells at d27 and found that STAT5CA cells outnumbered co-adoptively transferred control cells, reflecting better maintenance of Tcf1- compartment. Looking at cells expressing Cx3cr1, the researchers identified a population Texint cells, which were mostly derived from controls, and a similar, but distinct population of effector-NK-like cells, which was mostly composed of STAT5CA cells. These results suggested that Stat5a activation drove virus-specific CD8+ T cells into a distinct effector-NK-like state, rather than towards the typical exhaustion phenotypes.
Based on the enhanced accumulation and effector-NK-like features of STAT5CA cells, Beltra et al. evaluated their therapeutic potential in mice with established B16 tumors expressing the viral target antigen. While control cells only slightly delayed tumor growth, STAT5CA cells substantially decreased tumor burdens, resulting in enhanced survival in mice.
Evaluating the role of Stat5 in endogenous T cell responses, the researchers evaluated mice treated with Stat5iKO cells. After one month in a chronic infection setting, these cells were reduced compared to controls, and while they expressed PD-1 and Tox, most remained Texprog cells and failed to express molecules associated with Texint or Texterm states. This could not be rescued with PD-L1 blockade, suggesting that Stat5 is essential in the transition from the Texprog to Texint cell state. Similar results were observed with inducible deletion of Stat5a-b in mature Texprog.
Using CITEseq, the researchers were able to distinguish between Texprog1 and Texprog2 (quiescent, lymphoid tissue resident and proliferating, migrating progenitors, respectively), and found that Stat5a-b-deficient Texprog2 cells retained higher expression of progenitor-associated molecules that would typically be reduced during the transition from Texprog1. Further, the few Texint and Texterm cells that developed from Stata-b-deficient cells lacked expression of effector genes and killer lectin-like receptors, suggesting impaired re-entry into the cell cycle from quiescence and a failure to synthesize new proteins, resulting in defective differentiation.
Given the seemingly beneficial features associated with Stat5a expression, the researchers developed a strategy to take advantage of the Stat5a axis in a therapeutic setting though an orthogonal IL-2:IL2Rb system that allowed for selective targeting of Tex cells. In mice carrying adoptively transferred T cells transduced with the orthoIL2Rb-chain, treatment with orthoIL-2 specifically expanded those cells into Texprog2 and Texint cells, reducing the frequency of Texprog1 and Texterm cells in a dose- and Stat5a-b-dependent manner. This effect was enhanced when anti-PD-L1 was administered at the same time as orthoIL-2. Interestingly, this effect was more evident than when PD-L1 blockade was administered in the setting of Stat5a constitutive expression, suggesting that there is better synergy when the Stat5 activation and PD-1 blockade occur simultaneously.
Investigating whether the orthoIL-2:IL2Rb system could rewire established Tex cells, the team found that after 27 days in a chronic infection model, ortho-IL2Rb cells transferred into naive mice and treated with orthoIL-2 showed reduced Tox expression, robust expansion, with increased effector functions and polyfunctionality, especially in combination with anti-PD-1. ATACseq and analysis of transcription factor binding sites showed that these cells had distinct chromatin landscapes in which Teff and Tmem-like patterns were enriched, but epigenetic scars of exhaustion still remained.
Overall, these results show that while Tox programs exhaustion, Stat5a antagonizes Tox, playing a role in the transition of Texprog1 cells towards more differentiated exhaustion states. Enforced activation of Stat5a can directly inhibit Tox and subsequent exhaustion programming, instead inducing epigenetic remodeling that drives cells towards a durable effector NK-like state that is therapeutically useful, especially in combination with PD-1 axis blockade.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Jean-Christophe Beltra answered our questions.
What was the most surprising finding of this study for you?
We were very thrilled to see that manipulating Stat5 not only triggered a durable resistance of antigen-specific CD8+ T cells to exhaustion (even in the most stringent conditions), but also partially rescued cells that were already fully committed to the exhaustion lineage. Indeed, exhausted CD8+ T cells are epigenetically stable and, until now, few approaches capable of reversing this process have been identified.
The rescued CD8+ T cells were rewired towards a hybrid epigenetic state, displaying diminished exhaustion traits, partial restoration of effector/memory marks, and the acquisition of epigenetic marks unique to enhanced Stat5 activity. These findings present an exciting prospect: the creation of hybrid cell-types that, through precise manipulation of key signals, would incorporate the best attributes of each of the known developmental states (exhaustion, effector, memory), and would also foster unconventional features to achieve new cell states ideally equipped to eliminate cancer cells.
What is the outlook?
I consider this study along with other recent publications a pivotal proof of concept, affirming that the developmental trajectory of exhausted CD8+ T cells CAN be redirected by targeting specific signals (as demonstrated in our case for Stat5). While cytokine-based immunotherapies have proven efficient in the past, the use of these approaches has been constrained by severe off-target effects. However, the current blooming of sophisticated synthetic approaches and engineered tools now present an opportunity to direct cytokine-based therapy specifically to the desired cell type, potentially overcoming prior therapeutic limitations.
Empowering these new approaches with key fundamental discoveries on CD8+ T cell exhaustion holds promises for compelling translational applications. Of course, additional research is imperative to pinpoint the most efficient signals or, more likely, combinations of signals capable of reversing CD8+ T cell exhaustion and reigniting the full antitumor potential of these cells.
What was the coolest thing you’ve learned (about) recently outside of work?
Over the past few years, I have been traveling quite a lot, primarily for my research activities, often taking the opportunity to bring along my entire family. It is truly amazing to witness the impact those journeys have had on my children – how they have absorbed diverse cultures and cultivated a heightened sense of curiosity and reflection. I am also happy to have sparked in them a budding interest for adventures!




