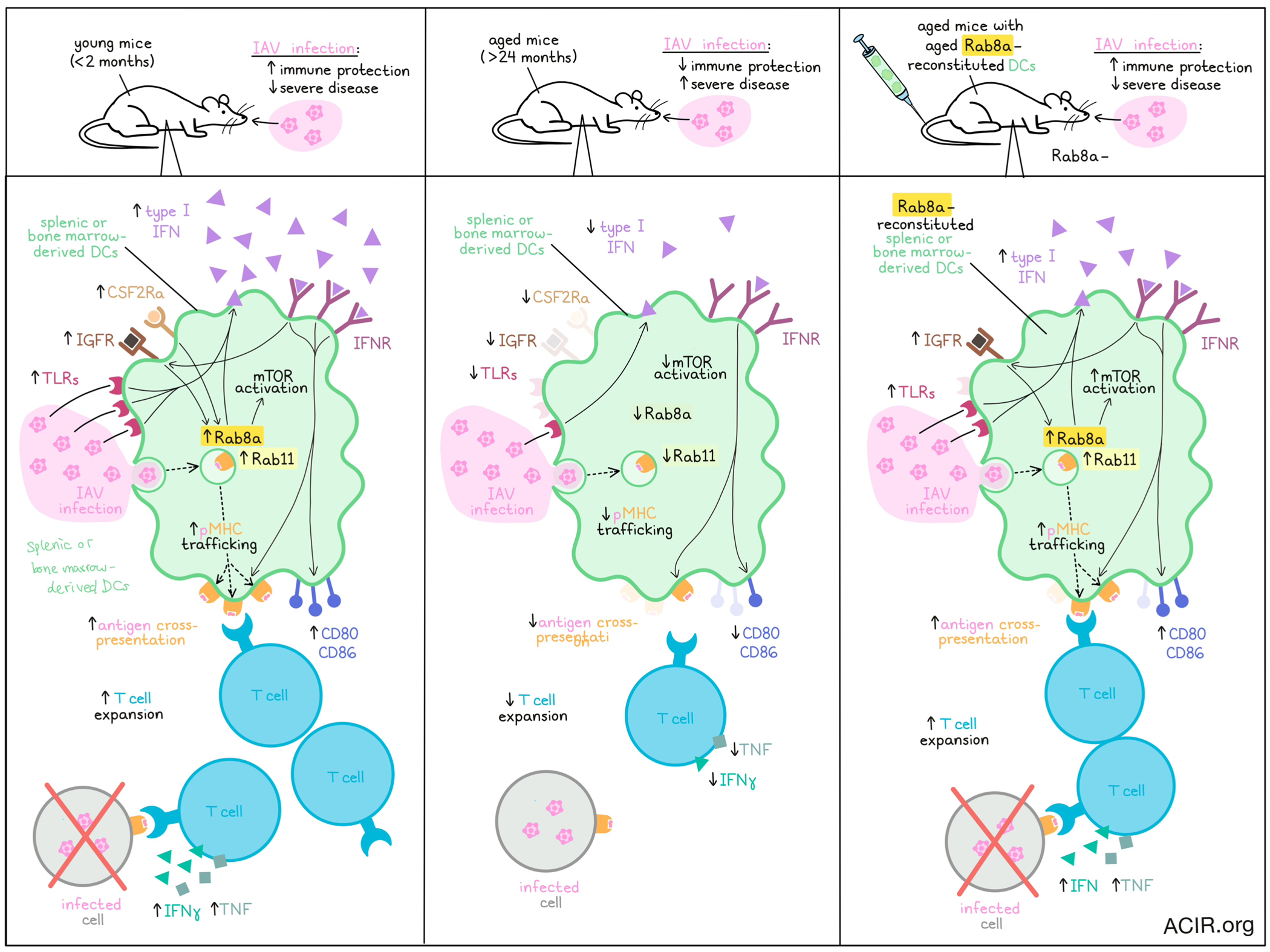
With advanced age comes impaired immunity and thus, increased susceptibility to diseases, including infections and cancer. Investigating factors that contribute to this reduced immune functionality, Singh et al. evaluated splenic and bone marrow-derived DCs and found that in aged mice, DCs showed reduced IFN responses, inefficient antigen presentation, and poor stimulation of adaptive immunity. This was found to be associated with reduced expression of Rab8a, and could be reversed with Rab8a overexpression. The results were recently published in Nature Communications.
To begin, Singh et al. isolated splenic CD19-CD3ε-CD11c+CD11b+ cells (representing DCs) from young (<2 months) and aged (>24 months) mice. Genome-wide transcriptomic analysis revealed that cells from aged mice showed upregulation of inhibitory molecules, molecules associated with NK cells, and several GTPase-associated nucleotide binding proteins, as well as downregulation of genes associated with antiviral responses, tissue remodeling, differentiation of Th1, Th2, and Th17 cells, functioning of NK cells, homing of immune cells, TCR signaling, cytokine signaling, IFN stimulation, and PI3K/MtorC1 signaling. Similarly, in GM-CSF and IL-4-induced CD11c+CD11b+ BMDCs, the researchers noted that aged mice had higher proportions of NK cells and downregulation of oligoadenylate synthase (OAS) genes, cell surface and endosomal TLRs, and pro- and anti-inflammatory cytokines. Cells from aged mice also showed reduced IFN responses upon viral stimulation, which was further evidenced by reduced expression of downstream JAK/STAT signaling molecules. Reduced IFN pathway genes were also confirmed in samples of PBMCs and CD11c+CD11b+-enriched PBMCs from aged (>65 years) versus young (<25 years) patients.
Given that IFNR signaling in antigen-presenting cells typically enhances the expression of MHCs and costimulatory molecules, Singh et al. investigated IFNR knockout mice. Like aged mice, IFNR KO mice had higher proportions of CD11c+CD11b+ cells, which less frequently expressed MHC-I, CD80, and CD86. Using OVA-pulsed BDMCs co-cultured with OT-I cells, the researchers showed that BDMCs from aged or IFNR KO mice were less capable of stimulating T cell responses. This was not due to reduced antigen uptake, but was instead found to be related to diminished cytokine sensing and a failure to upregulate Igf1r and Rab8a compared to cells from young mice. These results were mirrored in an adoptive cell transfer model in mice, in which transferred OVA-pulsed BMDCs from aged or IFNR KO mice (versus young mice) were less capable of inducing expansion of transferred OT-I cells, with fewer CXCR3+ OT-I cells (cells that preferentially migrate to inflammatory tissues) and fewer OT-I cells that produced IFNγ and TNF upon ex vivo stimulation. Further, when challenged with SIINFEKL-expressing Influenza A virus (IAV), mice carrying BMDCs from aged and IFNAR KO mice showed higher titres of replicating IAV and evidence of more severe disease, suggesting reduced immune protection.
Past research has shown that engagement of receptors CSF2Ra and IGFR lead to recruitment of the small GTPase Rab8a, which leads to activation of AKT via PI3K. As aged mice expressed lower levels of Csf2ra, Igf1r, and Rab8a, the researchers hypothesized that forcing overexpression of Rab8a might reconstitute some of the DC functionality that is lost in aged mice. Indeed, they found that Rab8a overexpression in bone marrow cells of aged mice induced upregulation of Akt1 and Bcl2 (involved in homeostasis) and resulted in a transcriptome resembling that of DCs from younger mice. Phenotypic and functional characterization further revealed that Rab8a overexpression increased expression of CD80, CD86, MHC-I, and TLRs, improved IFN responses upon stimulation with IAV, and better supported the expansion of OT-I cells. Similar results were observed in flt3L-induced BDMCs, which differentiate into cDC1s. Investigating whether Rab8a overexpression could restore DC antigen presentation, the researchers found that it did so more effectively when pulsing them with OVA rather than with SIINFFEKL peptides, suggesting that Rab8a may enhance antigen cross-presentation.
To further confirm the role of Rab8a in DCs, the researchers knocked it down using shRNA in young animals, and found that this reduced expression of Igf1r and downstream Akt1. Rab8a depletion in bone marrow cells of young mice reduced the frequencies of CD11c+CD11b+ BMDCs as well as those expressing CD80, CD86, MHC-I, and MHC-II. They also showed reduced differentiation and IFN response upon viral stimulation.
Next, Singh et al. used an OVA-pulsed DC vaccine model to evaluate whether Rab8a overexpression could enhance the functions of aged DCs in vivo, and found that like cells from young mice, CD11c+CD11b+ cells overexpressing Rab8a more frequently expressed CCR7 and CCR5 (associated with homing and migration), and induced stronger OT-I expansion in vivo, with OT-I cells making up a greater portion of T cells in various locations, and more OT-I cells capable of expressing IFNγ and TNF upon ex vivo stimulation. Upon challenge with SIINFEKL-expressing IAV after 1 month, mice treated with young or Rab8a-reconstituted aged CD11c+CD11b+ cells were better protected from infection, with lower viral loads and fewer signs of severe disease.
Investigating the mechanism by which Rab8a restores the functionality of aged DCs, Singh et al. noted that Rab8a-depleted bone marrow cells of young animals had significant changes in genes involved in the mTOR pathway, including reduced expression of Igf1r, Pi3kca, Akt1, Akt2, and Akt3, and increased expression of Tsc1 and Tsc2. Rab8a-reconstituted bone marrow cells, on the other hand, showed upregulation of Akt1 and pAkt at steady state, and these were further increased upon differentiation into BDMCs, suggesting that Rab8a promotes cell growth and survival via activation of the mTOR pathway. Rab8a-reconstituted BMDCs also showed increased peptide-loaded MHC-I molecules on their surface, suggesting that Rab8a could support the surface expression of membrane proteins, as Rab8a is associated with various membraneous compartments. Upon further investigation, the researchers found that Rab8a-reconstituted aged BMDCs upregulated Rab11, which further increased upon exposure to OVA. Compared to aged BMDCs, young or Rab8a-reconstituted aged BMDCs showed increased colocalization of Rab8a and Rab11, which also colocalized with H2-Kb-SIINFEKL upon OVA pulsing, together suggesting that Rab8a and Rab11 induce transport of pMHC-I complexes to endosomal recycling compartments, which facilitate trafficking of pMHC-I molecules to the plasma membrane for display on the cell surface.
Overall, these results show that while DCs in aged animals show downregulation of genes involved in differentiation, maturation, and functionality, their functionality can be partially restored with Rab8a expression, which enhances antigen presentation and supports the induction of adaptive immunity and immune memory. Restoration of the functionality of aged DCs could prove useful in therapeutic settings for both infection and cancer.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, lead author Sharvan Sehrawat answered our questions.

What was the most surprising finding of this study for you?
We aimed at analyzing dendritic cell (DC) functions in the aging host, given their ability to connect innate and adaptive immune response. Enhancing the functionality of DCs to achieve a favorable outcome in infection or tumorigenesis has received little attention, as most contemporary investigators focus on modulating the response pattern of adaptive immune cells. The most surprising finding was that a single molecules (Rab8a) could have such a dramatic phenotype, with the Rab8a-reconstituted aged BMDCs performing on par with the young cells, not only in inducing IFN response, but also in generating antigen-specific effector and memory response of cytotoxic T lymphocytes. This was probably due to its upstream role in the pathway following growth factor sensing by the receptors.
What is the outlook?
Key molecular details involved in the Rab8a-restored functioning of DCs in the context of immune aging are yet to be elucidated, and we are currently working on them. That the Rab8a-reconstituted aged DCs performed on par with, or even better than the cells from younger animals in some assays has led us to evaluate the utility of this approach in enhancing antitumor immunity in young animals as well. In fact, this is a new venture for us as the major focus of the lab is infection immunology! While some issues on the scalability of the “DC therapy” need to be resolved, we believe the approach would have broader applicability. This is because the immunodominant antigens are not always discernible for infectious agents or tumors of different types, which limits the utility of antigen-specific T cell based therapies.
What was the coolest thing you’ve learned (about) recently outside of work?
Providing direction to young minds in the laboratory is quite an enriching experience. Most of the research activities in Indian laboratories/institutes (IISER Mohali being no exception), are carried out by undergrads or the graduate students. It’s fun helping them become valuable scientists. In addition, I have recently learned how to quickly solve a Rubik’s cube! That was fun!!




