While it is known that both the innate and adaptive arms of the immune system follow circadian rhythms, the mechanisms behind these rhythms and their effects on vaccination treatment efficacy, particularly in the cancer setting, remain largely unknown. Two recent papers by Wang et al. in Nature and Cervantes-Silva and Carroll et al. in Nature Communications assessed these effects in more detail.
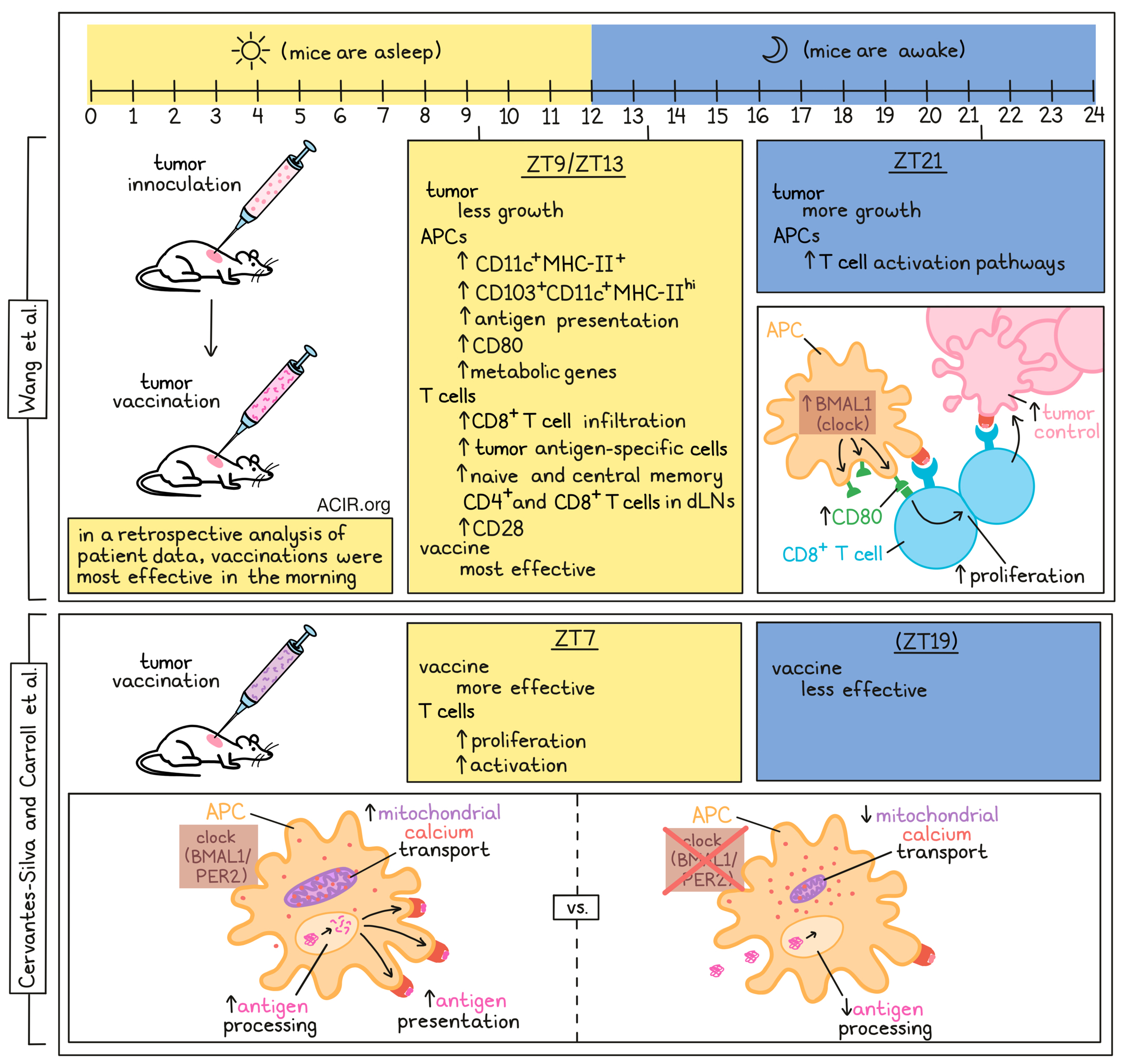
Wang et al. investigated whether the time of day of tumor inoculation affected tumor growth. B16F10 melanoma cells expressing ovalbumin (B16F10-OVA) were injected at six different times of day and tumor sizes were assessed over the course of two weeks. The different times of day were represented as Zeitgeber time (ZT), which is defined as the time following the onset of light in the facility, with ZT1 being 1 hour after light onset (morning) as the mice begin their period of rest. When tumors were injected late at night (ZT21), mice grew larger tumors, while injection in the late afternoon (ZT9 or 13) resulted in smaller tumors. These results were confirmed in three other tumor models and in the B16F10 model not expressing OVA, suggesting that antigen-specific immunogenicity was not the reason for the differences in growth.
To assess whether these tumor growth differences were caused by differences in antitumor immune responses, the same research was performed in NSG mice, which lack both adaptive and innate immune cells, and Rag2-/- mice, which lack only adaptive immune cells. In these models, no differences in tumor growth were detected when tumors were inoculated at different times of day. Assessing the immune infiltrate, the researchers found that CD8+ T cell infiltrates were higher in tumors that were inoculated during the day and lowest in those inoculated at night. Depletion of CD4+ or CD8+ T cells removed the time-of-day tumor growth effects, suggesting the adaptive immune response was responsible for the differences in tumor growth.
Tumors that were harvested four hours after tumor inoculation contained CD11c+MHC-II+ cells as the dominant immune cell subset, with the highest numbers in mice inoculated at ZT9. At a later time point (24hr), there were more central memory and naive CD4+ and CD8+ T cells, as well as CD11c+ subsets in the draining lymph nodes (dLNs) of mice inoculated at ZT9, compared to ZT21. Higher levels of OVA-presenting CD103+CD11c+MHCIIhi APCs and OVA-specific CD8+ T cells were present in dLNs of mice inoculated at ZT9. These data suggest there is increased antigen presentation and generation of tumor-specific T cells in dLNs of mice inoculated at ZT9.
When mice were used in which the circadian clock gene Bmal1 was deleted in T cells or in DCs, similar tumor volumes were reached after inoculation at ZT9 or ZT21. In these mice, there were fewer antigen-presenting DCs and antigen-specific T cells in the dLNs. RNAseq of CD11c+MHCIIhi migratory DCs obtained from dLNs after tumor inoculation in wild-type mice showed time-of-day differences in gene expression of clock and clock-controlled genes. Additionally, clusters that were more highly expressed in the ZT9 morning group were metabolic genes and the co-stimulatory molecule CD80, while the evening cluster was enriched for T cell activation pathways.
In vitro experiments with immature and LPS-matured bone marrow-derived DCs (BMDCs) that were synchronized using a serum shock, showed significant differences in Cd80 expression at different time points. Loss of BMAL1 revoked the Cd80 expression differences, suggesting that the circadian clock may regulate CD80 expression in DCs. Co-culture experiments showed that co-stimulatory signals were sufficient to promote time-of-day differences in T cell proliferation, while blockade of CD80 inhibited these differences.
Finally, the researchers assessed the effects on immunotherapy for cancer. Mice that were inoculated with B16F10-OVA cells at ZT9 were immunized with OVA at ZT9 or ZT21. Vaccination at ZT9 resulted in significantly more tumor volume reduction and higher levels of antigen-presenting DCs and CD4+ and CD8+ T cells in the dLNs. When vaccinations were performed in mice that had received tumors at ZT9 and ZT21, it was shown that the timing of the vaccination had a bigger effect on tumor growth than the timing of the tumor inoculation.
To see whether these results also translated to humans, human monocyte-derived DCs (hMoDCs) were synchronized in vitro. These cells expressed CD80 and the clock gene PER2. When the hMoDCs were pulsed with ELA peptide and co-cultured with ELA-specific CD8+ T cell clones from patients with malignant melanoma, there was a time-of-day difference in T cell proliferation. Finally, retrospective time-of-day analyses of a tumor vaccination trial showed that patients with melanoma who received the vaccine in the morning had higher levels of antigen-specific CD8+ T cells in the blood than those vaccinated in the afternoon.
In a separate study, Cervantes-Silva and Carroll et al. focused more deeply on the mechanism of circadian rhythm control in DCs in response to vaccination. Using a model for adoptive transfer, they isolated CD4+ T cells from OT-II mice at ZT3 and injected these into mice that had been phase-shifted to ZT7 or ZT19. The next day the mice were vaccinated with OVA and an adjuvant, and mediastinal lymph nodes were collected 72 hours later. There was an increase in T cell proliferation in mice vaccinated at ZT7, and these T cells were more activated (expressing CD69).
To assess whether antigen processing in DCs was regulated by the molecular clock, the researchers assessed BMDCs. These cells had the highest antigen processing at 12 and 36 hrs, and the lowest at 24 and 48 hrs after synchronization by serum shock. When Bmal1 was lacking in the BMDCs, cells expressed low levels of antigen processing, and no time-of-day effects were observed. In contrast, no differences in antigen uptake were detected at various times of day. Furthermore, splenic cDC1s, cDC2s, plasmacytoid DCs, and macrophages obtained at various time points during the day also showed antigen processing differences in vitro.
Given the known effects of the molecular clock on metabolism, the researchers then assessed the role of mitochondrial metabolism on these circadian effects in DCs. Knockout of Bmal1 demonstrated a rhythmicity in BMDCs and inhibitors of mitochondrial metabolism similarly impacted antigen processing in DCs, but only in Bmal1+/+ BMDCs. Next, the researchers found that circadian rhythm also affected mitochondrial morphology, a known indicator of mitochondrial metabolism, in DCs and this was mechanistically linked to their antigen processing ability. The changes in mitochondrial morphology and metabolism were affected by cellular calcium localization and calcineurin activity, which were controlled by the molecular clock. There was a circadian rhythm in gene levels of Bmal1 and Per2 in splenic DCs, as well as genes related to mitochondrial calcium uptake (Mcub and Emre). These data suggest that the antigen processing in DCs is regulated by the circadian control of mitochondrial calcium transport. When these processes were naturally inhibited, DCs were not able to process antigens, limiting response to vaccines at these time points.
Both groups showed that the time of administration of (tumor) vaccinations may impact vaccine efficacy. In particular, antigen processing and subsequent presentation and activation of T cells seem to be under influence of the circadian rhythm. Prospective trials in patients will be needed to study these effects in more detail and define the best time of day for the administration of vaccinations and other types of immunotherapy.
Write-up by Maartje Wouters, image by Lauren Hitchings.
Meet the researcher
This week, lead author Annie Curtis answered our questions.

What was the most surprising finding of this study for you?
To me, the most surprising result was realising that the clock is controlling mitochondrial morphology, such that it's changing the shape of the mitochondria from a tubular to a more fragmented configuration depending on time of day. It is remarkable that the circadian clock has such profound control on this organelle, and we have probably just scratched the surface as to the impact this is having on mitochondrial and dendritic cell function. There is evidence that the circadian clock is controlling the morphology of other organelles, such as the endoplasmic reticulum and lysosomes, which to me is wild and makes me wonder what impact that would have on immune cell function.
What is the outlook?
We already have evidence that this time-of-day effect for vaccine efficacy is indeed happening for influenza, SARS-CoV2, and the BCG vaccine. I would love to know if the mechanism we have uncovered in dendritic cells is responsible or what other mechanisms exist. Another study has elegantly shown that the dendritic cells migrate to the lymph node more readily at a certain time of day. In terms of translation, our study indicates that the best time to vaccinate might actually be in the middle of the night for humans, this may be tricky from a practical clinical standpoint, so we need to think about delivery systems that can accommodate this.
What was the coolest thing you’ve learned (about) recently outside of work?
From this project, I have been really impressed by peoples resilience and commitment. The two first authors on this study, Mariana and Richie had left the lab either last year or early this year and were busy with their own new careers and family. However they still spent many evenings over Zoom trying to work on the reviewers’ comments and the resubmissions (which was plural!). Also, it's been reinforced again to me that studies such as this can take a long long time, and that’s okay. The most important thing is that the science is correct and that we are making advances, however small, in the field.




