Since most cancer vaccines target antigens presented by MHC, the efficacy of such vaccines can be significantly impacted by the downregulation of antigen processing and presentation by tumor cells, which is a common feature in many cancer types. To circumvent this issue, Badrinath et al. developed a new vaccine strategy that enhances NK cell antitumor activity and is not dependent on MHC presentation for immune activation. The results of their preclinical analyses were recently published in Nature.
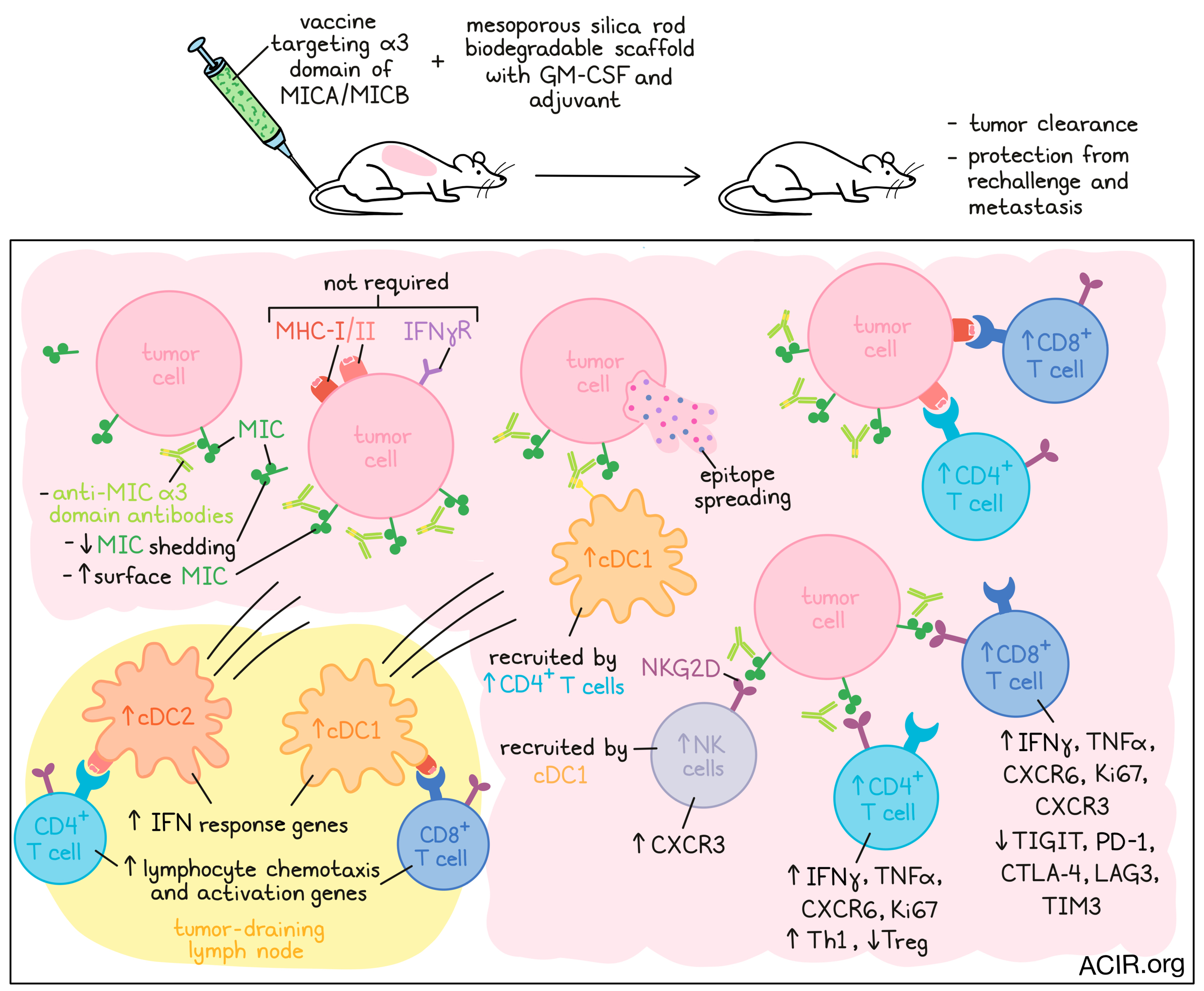
The researchers developed a vaccine that targets the MICA and MICB stress proteins, which are upregulated on cells upon DNA damage in cancer, but are rarely expressed in healthy cells. Binding of NKG2D to MICA/B activates cytotoxicity in NK and CD8+ T cells; however, tumors can evade this reaction by shedding MICA/B. Shedding reduces cellular expression levels of MICA/B, which induces NKG2D receptor internalization, resulting in NK cell function inhibition.
The vaccine targets the conserved α3 domain in MICA/B—the site of shedding—and was designed to trigger T and NK cell responses. The researchers also made use of a mesoporous silica rod biodegradable scaffold that contains GM-CSF and the adjuvant CpG ODN 1826 for vaccine delivery, which was shown to enhance the immune response.
Since the mouse NKG2D receptor binds human MICA/B proteins, MICB (or MICA) was expressed in mouse cancer cell lines for the in vivo experiments. Also, to provide a setting mimicking central tolerance, the human MICB gene was inserted transgenically into the host strain. Immunization with the MICB α3 domain vaccine induced MICB+ tumor-binding antibodies and anti-MICB CD4+ and CD8+ T cell responses. Small quantities of serum obtained from MICB-vaccinated mice inhibited MIC shedding and increased MICA/B protein levels on cell lines. Stabilization of MIC was also detected in vivo, where it resulted in increased MICB surface expression on B16F10 tumors.
The vaccine was effective against subcutaneous B16F10 and EL4 tumors expressing MICA or MICB and provided complete protection from tumor rechallenges, even four months after immunization. To test the efficacy of the vaccine in a metastatic setting, mice were immunized after surgical removal of primary tumors in the B16-BL6 (MICB) melanoma and the 4T1 (MICB) triple-negative breast cancer models, which are prone to metastases. Vaccination reduced the number and size of lung metastases one month after surgery.
To further assess the mechanisms of action of the vaccine, the aggressive B16F10 (MICB) melanoma model was used to study the tumor microenvironment (TME). Tumors of vaccinated mice contained a high percentage of effector lymphocytes, while the Treg ratio among CD4+ T cells decreased. CD4+ and CD8+ T cells expressed higher levels of NKG2D and CXCR6, as well as increased levels of IFNγ, TNFα, and Ki67. In contrast, expression of PD-1, CTLA-4, TIM3, TIGIT, and LAG3 was lower on CD8+ T cells from vaccinated tumors than in controls. These effects were not seen when, instead of the vaccine, mice were treated with anti-MICA/B antibodies directed against the α3 domain.
scRNAseq analysis showed that myeloid cells were most abundantly present in the control groups, while T cells and NK cells dominated in tumors of the vaccinated group. TCRseq analysis revealed clonal expansion of both CD4+ and CD8+ T cell populations in the vaccinated group. CD4+ and CD8+ T cells had increased levels of activation-related genes, CD4+ T cells upregulated Th1 signature genes, CD8+ T and NK cells had increased expression of Cxcr3, and both CD4+ and CD8+ T cells had higher levels of Cxcr6. Additionally, ILC1s, Xcl1+ NK cells, and cytotoxic NK cells expanded. Depletion experiments showed that CD4+ and CD8+ T and NK cells played important roles in vaccine efficacy, with CD4+ T cells being essential for its effects.
To test whether the vaccine was also effective in the setting where antigen presentation in tumor cells is affected, the researchers tested the vaccine in B16F10 (MICB) tumors that had a loss of MHC-I (B2m-/-), MHC-II (H2-Aa-/-), or IFNγ receptor (ifngr1-/-). In these settings, the vaccine was efficacious, with 50-75% of mice remaining tumor-free at day 100. Depletion experiments showed that CD4+ T cells and NK cells were essential for efficacy in the B2m-/- model, and CD4+ T cells were required for NK cell influx.
To further assess how CD4+ T cells affect NK cell infiltration, Badrinath et al. investigated dendritic cell (DC) subsets in the TME. Vaccinated mice had higher levels of migratory cDC1 and cDC2 in the tumor-draining lymph nodes (TDLN), an effect that was abolished when CD4+ T cells were depleted or CD40 ligand was blocked. These TDLN DCs upregulated genes associated with interferon response and lymphocyte chemotaxis and activation. In the tumor, vaccinated mice also had higher levels of cDC1 than controls. To assess the role of cDC1 in NK cell recruitment to tumors, Xcr1DTR mice in which cDC1 can be specifically depleted using diphtheria toxin were used. Removal of DCs decreased the tumoral infiltration of NK cells and CD4+ and CD8+ T cells.
To assess whether the vaccine-induced anti-MICB antibodies affected DC function, DCs were cocultured with irradiated B16F10 (OVA) tumor cells with a B2m mutation, prohibiting antigen presentation to OT-1 CD8+ T cells. Purified serum from immunized mice enhanced CD8+ T cell proliferation in these cultures, suggesting that the antibodies could enhance the response to other tumor cell antigens. CD8+ T cells isolated from MICB-vaccinated mice bearing B16F10 (MICB) melanoma proliferated in vitro in response to the gp100 melanoma peptide. Additionally, gp100-specific pmel-1 T cells proliferated in the TDLN of immunized mice. This effect was reduced when cDC1s were depleted before the transfer of pmel-1 T cells. Finally, DCs isolated from the TDLN of immunized mice induced pmel-1 CD8+ T cell proliferation in vitro in absence of antigen.
Finally, the researchers then moved to test the safety and immunogenicity of the vaccine in rhesus macaques. The macaque MICA/B ɑ3 domain was used for vaccination to maximize the detection of any toxicities. Immunization resulted in the production of anti-MICA and anti-MICB antibodies, and titers could be substantially increased with a booster vaccine. No clinical adverse events were observed in response to immunization.
Therefore, the MICA/B vaccine induced T and NK cell immunity, prevented MICA/B shedding, and led to increased antigen cross-presentation and epitope spreading. Given the high efficacy of this vaccine in mouse models, and its safety in primates, a clinical trial with this vaccine is being planned.
Write-up by Maartje Wouters, image by Lauren Hitchings.
Meet the researcher
This week, first author Soumya Badrinath answered our questions.

What was the most surprising finding of this study for you?
Recent clinical data demonstrate that patients who are refractory to checkpoint inhibitors often harbor loss-of-function mutations or downregulate genes related to MHC-I antigen presentation or IFNγ signaling pathways. Mutations in these genes greatly impair CD8+ T cell-mediated immunity. We were excited to find that the MICA/B vaccine was highly effective in controlling tumors that were deficient in MHC-I (B2m-/-)- or IFNγ receptor (Ifngr1-/-), with 50-75% of vaccinated mice remaining tumor-free beyond day 100. Another surprising finding was that in addition to the polyclonal antibodies, CD4+ T cells were critical for the vaccine efficacy. CD4+ T cells played an important role in recruiting diverse effector T cell and NK cell populations to the tumors. Vaccine-induced immunity against MHC-I-deficient tumors was mediated by the coordinated action of both CD4+ T cells and NK cells.
What is the outlook?
We have made great progress with peptide vaccines for the treatment of cancers. This approach, however, requires personalization due to the vast inter-individual diversity of MHC molecules. Our vaccination strategy and design allows it to be used more generally across a large patient population whose tumors express MICA/B. The vaccine engages multiple effector cells, thus enabling elimination of tumor cells that arise as a result of acquired resistance.
Many patients undergoing surgical removal of primary tumors already have established micro-mets that are hard to detect. MICA/B vaccine could prevent recurrence of metastatic disease in such patients. This vaccine could also be readily combined with local radiation therapy, since DNA damage enhances MICA/B expression by tumor cells.
What was the coolest thing you’ve learned (about) recently outside of work?
Outside of work, I have learned a lot about plants and fishes recently. We have around 45 indoor plants and 3 aquariums (along with 2 golden retrievers and a baby). I love gardening, and we have been growing our own vegetables every summer for the past few years. This year, we are growing cabbage, broccoli, cauliflower, Brussel sprouts, peppers, eggplant, okra, beans, spinach, amaranth, curry leaves, banana plant, moringa, beets, kohlrabi, tomatoes, squashes, ridge gourd, bitter gourd, peas, cucumbers, various berries, flowers, and herbs. I find that gardening in many ways is very similar to my life as a researcher – it requires a lot of dedication, effort, and patience!





