Recent advances in IL-2 treatment strategies have led to the development of IL-2Rβγ-biased approaches in which toxicity is expected to be lower. However, recent clinical research has shown disappointing results. Wu, Chia, et al. assessed the mode of action of various IL-2 constructs to help the future design of IL-2 treatments. Their results were recently published in Nature Cancer.
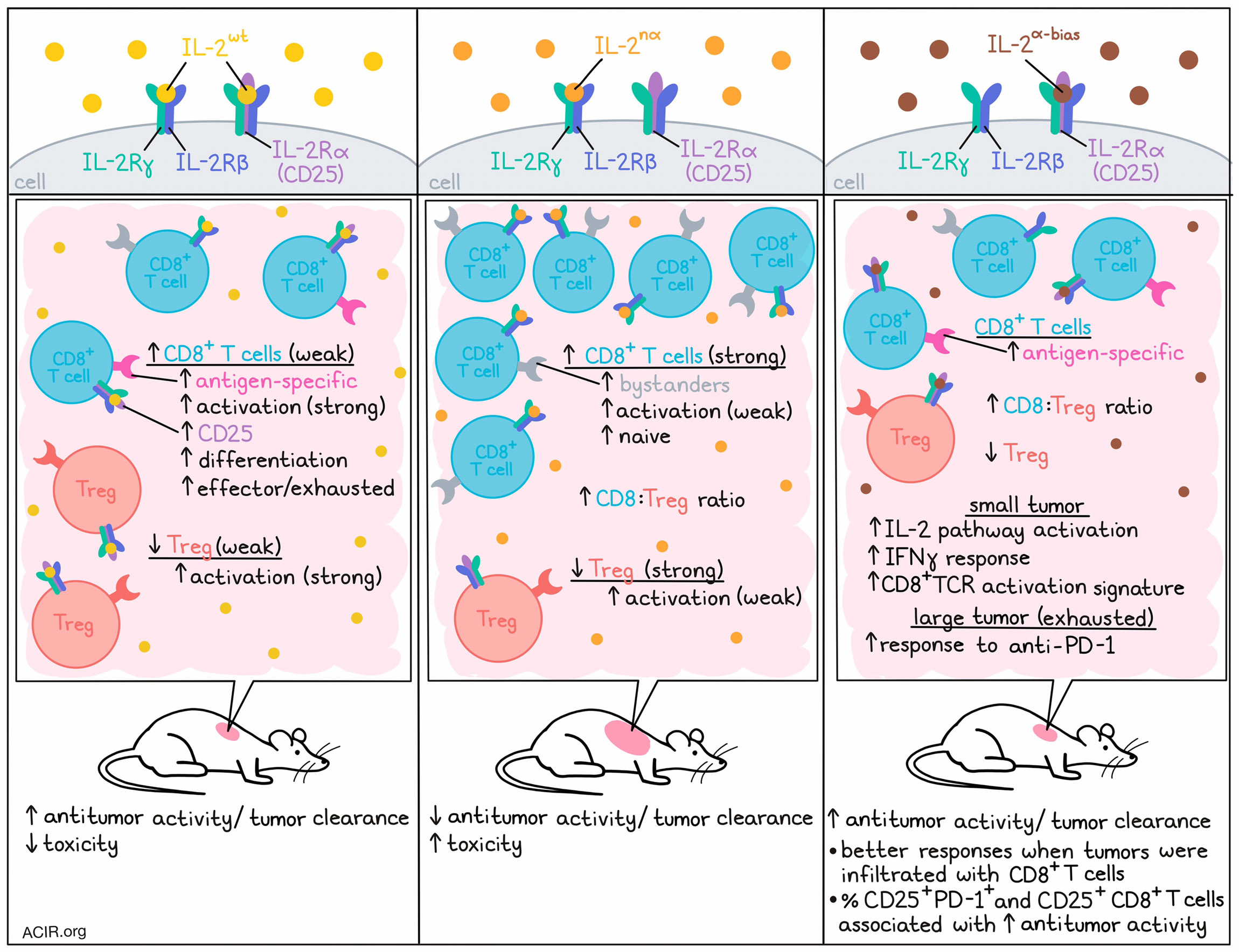
To investigate the mode of action of various forms of IL-2 treatment, the researchers tested human IL-2wt with IL-2nα (which has IL-2Rα-disrupting mutations resulting in an IL-2Rβγ-biased IL-2 analog). In vitro, IL-2wt activated Tregs more efficiently than IL-2nα, while both versions had similar activity on resting CD8+ T, CD4+CD25- T, and NK cells (which all express IL-2Rβγ). However, in vivo, IL-2wt had stronger antitumor efficacy and resulted in lower body weight loss and fewer deaths than IL-2nα. This was surprising, as previous data suggested that IL-2wt would favor Tregs in the tumor microenvironment (TME), resulting in immunosuppression, and binding to IL-2Rα on endothelial cells would result in toxicity.
Treatment with IL-2nα induced higher CD8+ T cell-to-Treg ratios than IL-2wt in both tumors and peripheral tissue. However, the increased numbers of CD8+ T cells did not result in better antitumor responses. As tumor-infiltrating lymphocytes (TIL) in the TME can be tumor antigen-specific effector T cells (TST) and tumor-irrelevant bystander T cells, there might be differences in the activation of TST and bystander cells by these different IL-2 constructs. IL-2wt was found to expand more CD39+CD8+ T cells or p15E-tetramer+CD8+ T cells in the TME, while IL-2nα expanded CD39-PD-1- bystanders. TST cells expressed higher CD25 levels than bystander cells and were more likely to be activated by IL-2wt.
The researchers then performed single-cell RNA sequencing (scRNAseq) on immune cells isolated from tumors treated with the two constructs. Zooming in on the T cell populations, eight clusters could be observed. IL-2wt increased the number of activated CD8+ T cells and conventional CD4+ T cells, while IL-2nα expanded naive CD8+ T cells. Both treatments decreased the proportion of Tregs, but this was more strongly the case in the IL-2nα group. Trajectory and gene expression analyses showed that IL-2wt stimulated the differentiation of CD8+ T cells into more activated, effector, and exhausted states, while IL-2nα kept a less activated and naive T cell phenotype.
The researchers then created IL-2α-bias, in which IL-2-IL-2Rβ binding was interrupted. This construct could bind IL-2Rα and had activity on CD25-expressing T cells only. To test whether selective activation of CD25+CD8+ effector T cells was sufficient for antitumor activity, MC38 tumor-bearing mice were treated with IL-2α-bias. Treatment effectively inhibited tumor growth, and in B16F10-OVA tumors it increased the proportion of the OVA-tetramer+CD8+ TILs.
The researchers then assessed how treatment with IL-2α-bias affected Treg populations. In the peripheral blood, treatment increased the proportion of Tregs and downregulated the percentage of CD8+ T cells. However, in the tumor an opposite trend was observed, with an increase in the proportion of CD8+ T cells and decrease of Tregs, suggesting the effects of IL-2α-bias are context-dependent.
Given that expression of CD25 and PD-1 is regulated by TCR signaling, the researchers hypothesized that a population of tumor antigen-experienced CD8+ TILs coexpress CD25 and PD-1, and serve as the major antitumor immune cells. In CD8+ TIL from MC38 tumors, most cells expressing CD25 also expressed PD-1, while PD-1+CD25+CD8+ T cells were mostly absent in the spleen. In various tumor types, the percentage of PD-1+CD25+ cells among the total CD8+ TIL population varied. Tumors with relatively higher CD8+ T cell infiltration (MC38, B16F10, and CT26) responded better to IL-2α-bias treatment than those with lower TIL, and the percentage of PD-1+CD25+ and CD25+ cells among CD8+ TILs strongly correlated with antitumor activity in these models.
To assess whether the PD-1+CD25+CD8+ TIL subset also exists in human tumors, scRNAseq data of CD8+ T cells obtained from 14 tumor collections and matched normal tissue were compared. Coexpression of PD-1 and CD25 was detected in 0.2-5.2% of all CD8+ TIL across different tumor types and these cells were more prevalent in tumors than in healthy tissues.
To determine how anti-PD-1 treatment may affect IL-2 signaling, in vitro experiments were performed with human T cells. Anti-PD-1 treatment stimulated an increase in production of IL-2 by stimulated T cells, which was mainly coming from the PD-1+CD25+CD8+ population. Returning to mice, in MC38 tumors, treatment with anti-PD-1 stimulated IL-2 production in CD25+PD-1+ and CD25+PD-1-CD8+ T cell subsets.
To further examine how IL-2 affects response to anti-PD-1 treatment, Wu, Chia et al. reanalyzed biomarker and efficacy data from a clinical trial assessing the anti-PD-1 antibody sintilimab plus pemetrexed and platinum (chemo) in patients with non-squamous non-small cell lung carcinoma. In those treated with the combination, there was a strong positive correlation between the IL-2 pathway signature and tumor responses, with those with a higher signature having better responses and improved survival. These results were confirmed in another study with data from patients with melanoma treated with various anti-PD-1 antibodies. Therefore, the IL-2 signature may serve as a biomarker for therapy response in PD-1-targeting therapies.
To assess the effects of IL-2 signatures in tumors with T cells in exhausted states, the researchers developed mouse models with different exhaustion states. In mice with small, less exhausted tumors, anti-PD-1 resulted in tumor regression, while in large tumors with fixed dysfunctional states, the effects were limited. Bulk RNAseq showed that small tumors had higher IL-2 pathway activation, IFNγ response, and CD8+TCR activation signatures than large tumors. In both TIL-exhausted MC38 and TIL-excluded EMT6 large tumor models, IL-2α-bias had a synergistic effect when combined with anti-PD-1, resulting in eradication of most tumors, long-term survival, and protection against tumor rechallenge.
This study suggests that IL-2 targeting CD25 can specifically activate tumor-reactive T cells instead of increasing the number of bystander T cells in the tumor, and that treatment may work synergistically with anti-PD-1 treatment. Therefore, these data can inform the development of more effective IL-2 therapies.
Written by Maartje Wouters, image by Lauren Hitchings.




