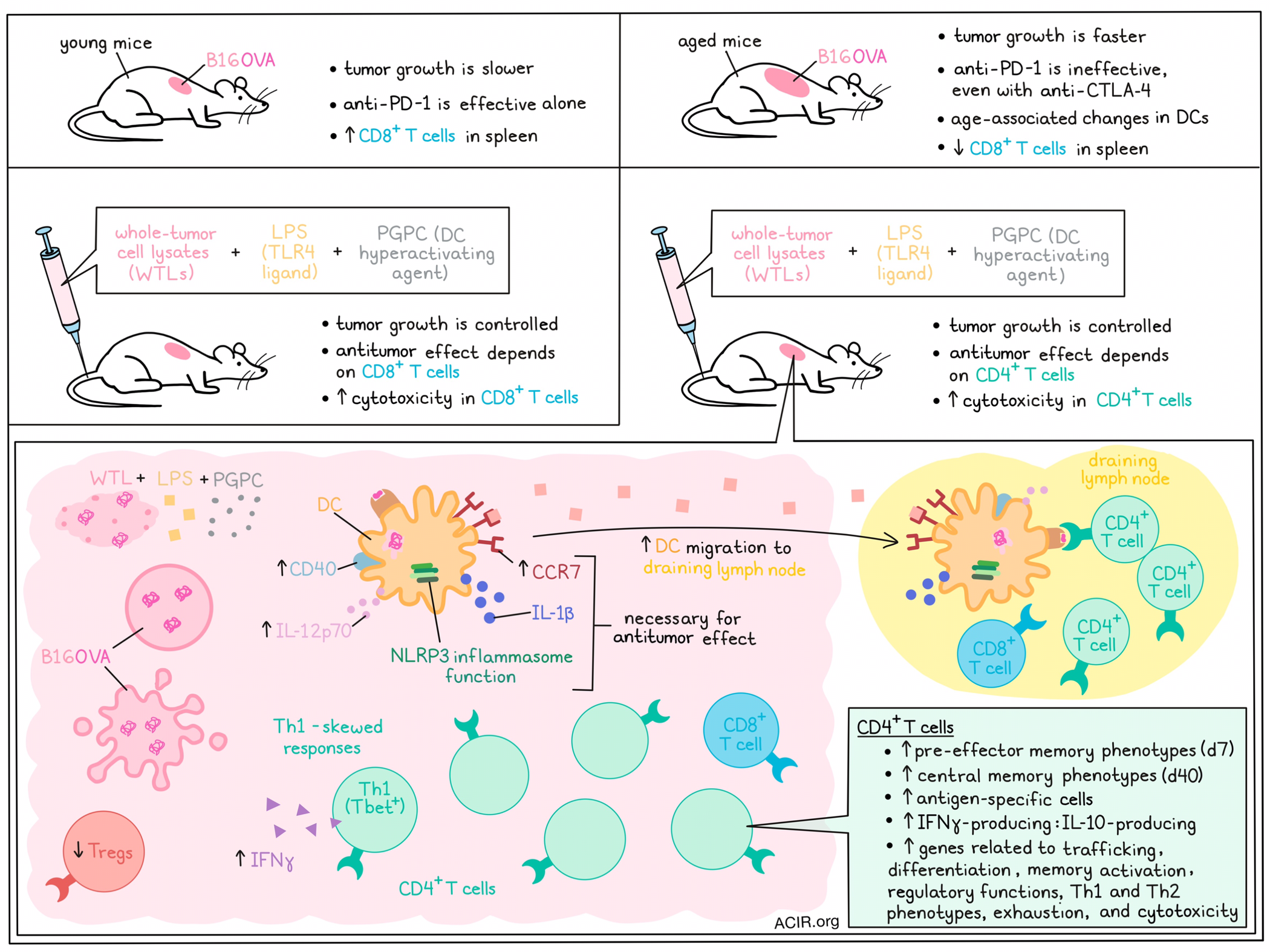
Aging results in decreased immune system functionality, with age-associated changes to dendritic cells (DCs) limiting their migration and cytokine production. Given that such effects may induce immunotherapy resistance, Zhivaki et al. investigated a strategy with vaccine adjuvants to overcome these changes to DCs in murine models. Their research was recently published in Cell.
The researchers began by assessing antitumor responses to B16OVA murine melanoma tumors. Tumor growth was similar in mice aged 8, 20, and 40 weeks, but tumor growth was faster in 68- and 92-week-old mice. To determine how age impacts immune checkpoint blockade (ICB) efficacy, mice were treated with anti-PD-1 or anti-PD-1/anti-CTLA-4. Anti-PD-1 alone improved survival and reduced tumor growth in 8-, 20-, and 40-week-old mice, while anti-PD-1 was ineffective in mice aged 68 or 92 weeks, even with the addition of anti-CTLA-4.
Zhivaki et al. then investigated whether vaccine adjuvants that induce DC hyperactivation could overcome these issues. Vaccines consisting of whole-tumor lysates (WTLs) of B16OVA and adjuvants for DC stimulation: the TLR4 ligand lipopolysaccharide (LPS), LPS + Alum (FDA-approved adjuvant), or LPS + 1-palmitoyl-2-glutaryl phosphatidylcholine (PGPC; to induce DC hyperactivation) were then assessed in young (8 weeks) and elderly (68 weeks) mice. The “hyperactivator” adjuvant (LPS + PGPC) resulted in tumor rejection in approximately 80% of mice of both ages, while the other vaccination strategies were not effective in aged mice.
Older naive mice had a lower percentage of CD8+ T cells in the spleen, and while the induction of tumor rejection after WTL + LPS + PGPC immunization was dependent on CD8+ T cells in young mice, it was dependent on CD4+ T cells in elderly mice. This prompted assessment of age-dependent CD4+ T cell functions after vaccination. Mice aged 8 or 90 weeks were immunized with OVA + LPS + PGPC, and scRNAseq was performed on CD3+ T cells from skin draining lymph nodes (dLNs). In immunized aged mice, genes related to T cell trafficking, T cell differentiation, memory T cell activation, and a regulatory signature were upregulated in CD4+ T cells. Further, Th1- and Th2-related genes were enriched in CD4+ T cells from elderly mice, as well as exhausted cells and the cytotoxicity program score.
The cytotoxicity of spleen CD4+ and CD8+ T cells obtained after immunization was assessed by coculture with B16OVA cells. CD8+ T cells from young mice showed cytotoxicity against tumor cells, while CD4+ T cells from immunized aged mice were cytotoxic, suggesting DC hyperactivation induced CD4+ T cell antitumor responses.
In DCs, NLRP3 inflammasome function, IL-1β, and CCR7 were all found to be necessary for the antitumor responses in elderly immunized mice. To assess the hypothesis that DC hyperactivating vaccines result in antitumor responses in elderly mice by correcting age-associated DC function defects, the activity of DCs from mice of various ages was assessed. Some DC activities that were corrected with the DC hyperactivator included induction of CCR7 and CD40 expression on DCs, and IL-12p70 secretion by DCs. To assess if the CCR7 recovery resulted in differences in LN migration, CFSE-stained bone marrow-derived DCs (BMDCs) were injected into young and elderly mice, which revealed that LPS-treated elderly DCs could not migrate to the dLN, while treatment with LPS + PGPC resulted in increased DC migration to the dLN.
DC hyperactivation resulted in Th1-skewed T cell responses. To determine if this ability of hyperactive DCs to induce Th1-skewed responses also applied to antigen-experienced (memory) T cells, OVA-specific T cells were generated by immunizing mice with OVA. CD4+ T cells were isolated and cultured with OVA-loaded BMDCs, which were differentially stimulated with DC agonists. Compared to other stimuli, LPS + PGPC induced most IFNγ expression in Th1 (Tbet+) T cells.
The researchers then investigated whether the enhanced Th1 activities could be explained by differential T cell differentiation after immunization. At 7 and 40 days after immunization, memory and effector T cells in dLNs were assessed. At 7 days, hyperactivation of DCs induced CD4+ pre-effector memory T cells, and at 40 days, central memory T cells were detected, and both these populations were less abundant in mice receiving the immunizations without PGPC. To assess the role of DC inflammasomes, mice with WT or Nlrp3-/- DCs were generated and immunized. At 7 days, mice with Nlrp3-/- DCs, had lower levels of pre-T effector memory cells, and lower levels of OVA-specific CD4+ T cells in the dLN and spleen than WT mice, suggesting NLRP3 is important for the T cell differentiation effects.
The researchers then assessed the longevity of the induced T cell responses. Young mice were immunized and boosted two weeks later. Sixty weeks after immunization, mice received an OVA boost and were implanted with B16OVA cells. Only the WT mice immunized with OVA + LPS + PGPC rejected the tumors and had the highest levels of OVA-specific CD4+ T cells. These effects were found to be dependent on NLRP3. To determine if the T cells generated by hyperactivated DCs were able to elicit protective immunity, isolated immune cells from 69-week-old mice which had been immunized at week 8, were injected into naive 8-week-old mice. One week after the transfer, mice were injected with B16OVA cells. CD4+ T cell transfer protected against tumor growth in 80% of mice, suggesting long-lived memory CD4+ T cell responses were responsible for the antitumor immunity. The immunization of Nlrp3-/- mice did not induce antigen-specific T cells responses that persisted to old age.
The researchers assessed the antitumor function of CD4+ T cells in the tumors of immunized 68-week-old mice with B16OVA tumors. Fifteen days after immunization, there was a decrease in the proportion of Tregs and an increase in the ratio of IFNγ-producing to IL-10-producing CD4+ T cells in the TME, compared to untreated mice.
To confirm that the hyperactivated DC state could also be reached in human DCs, human monocyte-derived DCs (moDCs) were generated and stimulated with the DC stimuli. After 24 hours, the moDCs treated with LPS + PGPC released IL-1β and produced IL-12p70, while IL-10 was absent, suggestive of a Th1-skewed pattern. Further, these moDCs induced IFNγ production in allogeneic naive T cells. When moDCs were generated from individuals aged >70 years, IL-1β was produced, and this was sensitive to an NLRP3 inhibitor. Finally, transwell experiments with elderly moDCs showed that stimulation with LPS + PGPC induced higher levels of migration.
Therefore, this research shows that age-related DC dysfunction can be overcome by adjuvants that induce DC hyperactivation, inducing CD4+ T cell antitumor responses. This opens up new avenues into research for combination immunotherapy strategies to overcome therapy resistance, particularly in older individuals, who make up the bulk of the population affected by cancer.
Write-up by Maartje Wouters, image by Lauren Hitchings




