Surgery, a common treatment option for high-grade glioma, is mostly ineffective long term, however, the resected tumor could serve as a source of antigens for the development of personalized vaccines that could improve long-term outcomes. Field et al. had previously created a vaccine comprised of irradiated glioma cells pulsed with the glycolipid α-galactoceramide (α-GalCer) and tested it in a glioma mouse model. α-GalCer is presented by antigen-presenting cells to natural killer T (NKT) cells via an MHC-I-like molecule CD1d. As CD1d is non-polymorphic and the T cell receptor of NKT cells is mostly invariant, α-GalCer is an attractive immune adjuvant with broad applicability. Although the vaccine prevented tumor development when administered prophylactically, it did not result in long-term survival benefit in a therapeutic setting unless Treg suppression was also addressed. In the current paper, published in OncoImmunology, Field et al. explored adding anti-CTLA-4 just before or close to vaccine administration in an orthotopic mouse model of glioma to target the T cell priming phase and enhance the antitumor response initiated by the vaccine.
In the prophylactic setting, the α-GalCer-pulsed, irradiated GL261 glioma cell vaccine, intravenously injected seven days before subcutaneous GL261 tumor challenge, resulted in significant protection against tumor development. However, when the vaccine was administered therapeutically seven days after tumor challenge, the tumor was not eradicated.
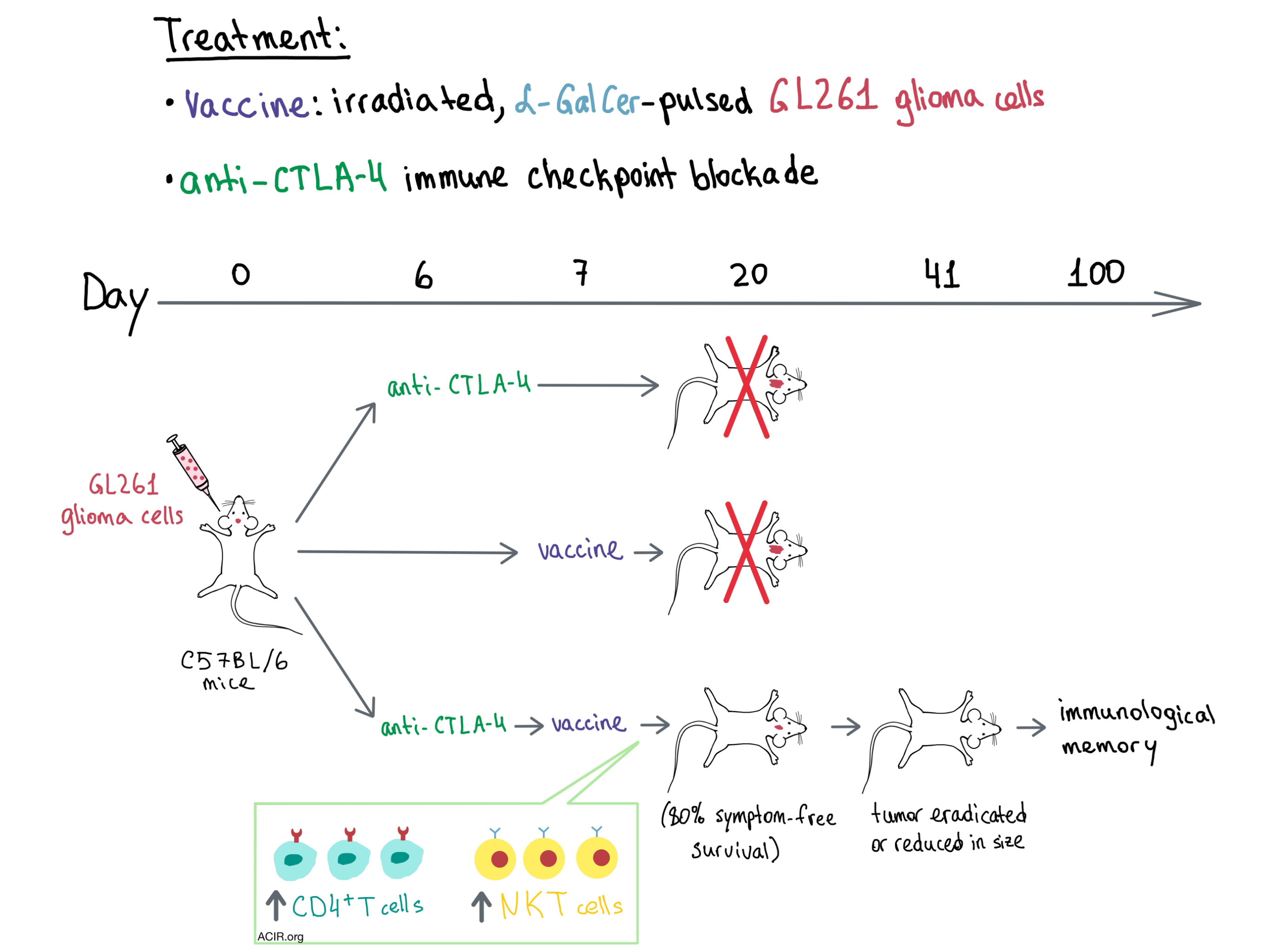
To test whether the addition of immune checkpoint blockade would improve the outcome in the therapeutic setting, the team administered a single dose of anti-CTLA-4 either one day before vaccination or three days after. In both cases, the tumors initially grew, but then completely regressed. Tumor growth was lower when anti-CTLA-4 was administered prior to vaccination.
Moving on to the intracranial tumor setting, neither the vaccine nor anti-CTLA-4 alone had any positive effect on survival, but a single dose of anti-CTLA-4 before vaccination resulted in a significant antitumor response, prevented the onset of tumor-related symptoms in most mice, and significantly prolonged symptom-free survival, with long-term survival averaging 80%. Administering anti-CTLA-4 3 or 7 days after vaccination was significantly less effective, implying that checkpoint blockade was most helpful during immune priming. Mice with complete response rejected subcutaneous tumor challenge 100 days after initial intracranial tumor establishment, indicating the presence of immunological memory. Tumor regression and increased immune cell infiltration into the tumor following combination treatment were confirmed radiologically and histopathologically.
To identify the cellular players involved, the researchers took a closer look at the immune infiltrates in the spleens, lymph nodes, and brains of tumor-bearing mice. Within the spleen and lymph nodes, early timing of CTLA-4 blockade significantly enhanced proliferation of CD4+ and CD8+ T cells (but not Tregs), with the greatest increase observed with combination treatment. CD4+ T cells expanded more than CD8+ T cells, and tumor-specific cells were highly prevalent in the immune infiltrate. In the brain, statistically significant increases in the number of immune cells were only observed with combination treatment, with the majority of the infiltrate consisting of macrophages, microglia, and lymphocytes. Within the T cell compartment, CD4+ T cells were highly represented, while the numbers of CD8+ T cells and Tregs did not change significantly. Examination of the TCR repertoire in the brain revealed that the combination treatment resulted in increased infiltration of diverse oligoclonal T cell populations, although the antigen-specificity of these clones was not determined. Overall, the team discovered that the therapeutic effect of vaccine and anti-CTLA-4 combination treatment was dependent on CD4+ T cells and activated NKT cells. How CD4+ T cells mediate tumor cell elimination in this setting is yet to be determined.
Field et al. demonstrate that early, but not late, administration of anti-CTLA-4 close to immune priming with an α-GalCer-pulsed whole cell vaccine confers long-term survival benefit and tumor regression in an orthotopic glioma mouse model. The researchers hypothesize that using anti-CTLA-4 in this temporally restricted manner rather than the extended multi-dose schedule currently utilized in the clinic, may also avoid the autoimmune side effects commonly observed with this immune checkpoint blockade.
by Anna Scherer




