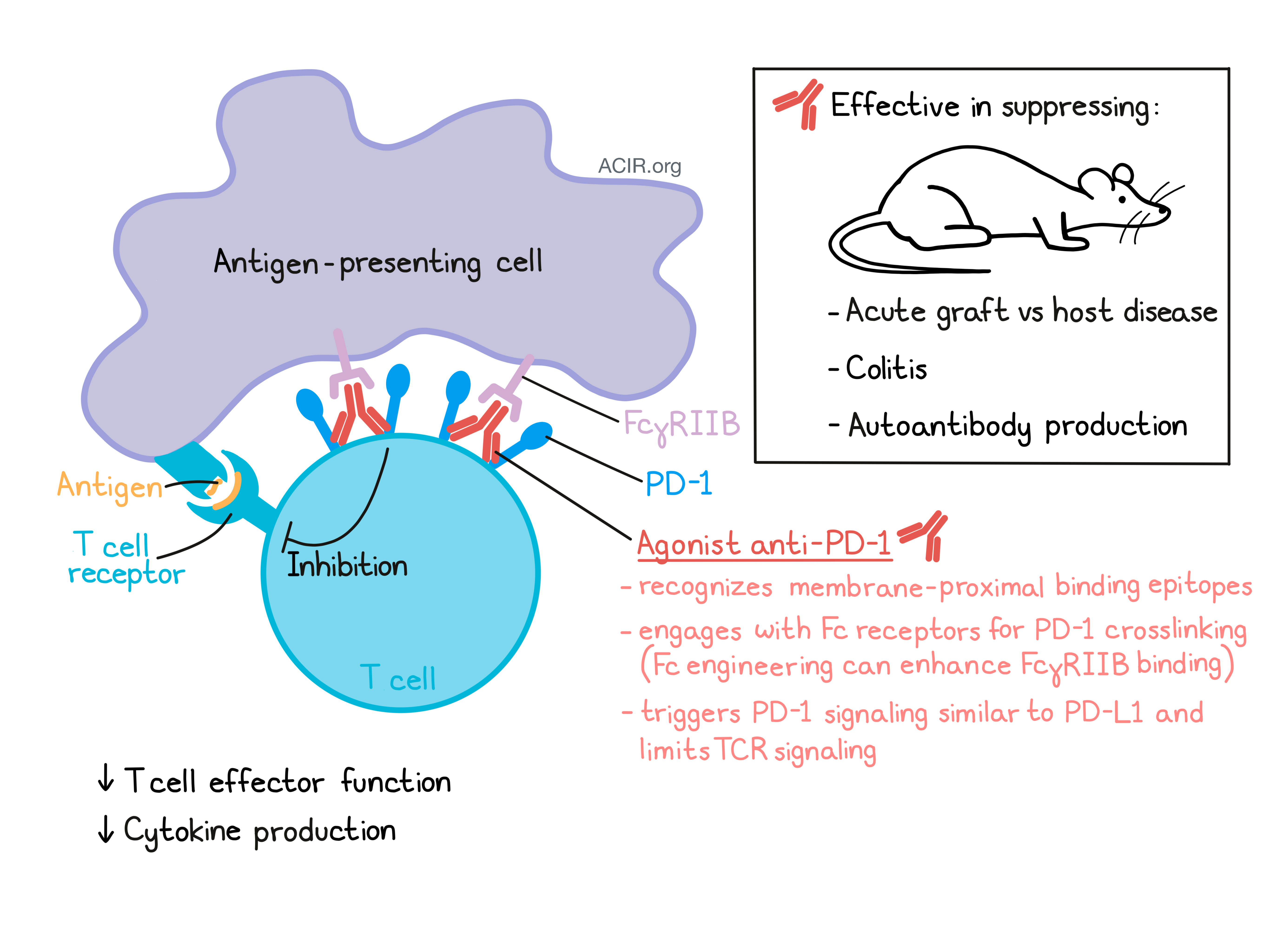
Antibodies blocking the PD-1/PD-L1 interaction are widely used to enhance immune responses against cancer. This group of antibodies binds to the membrane-distal region of the PD-1 molecule. In a recent paper published in Science Immunology, Suzuki and Tajima et al. identified another group of anti-PD-1 antibodies recognizing the membrane-proximal extracellular region of PD-1. These antibodies act as agonists, suppress inflammation in mouse models, and may have potential as drugs for the treatment of various inflammatory diseases, including autoimmune diseases.
Setting out to define the requirements for antibody molecules to stimulate, rather than inhibit PD-1, Suzuki and Tajima et al. first confirmed that colocalization and co-ligation of PD-1 and the TCR by immobilized PD-L1-Fc, and anti-CD3 and anti-CD28 antibodies was necessary to suppress T cell function. However, co-ligation of PD-1 and TCR with immobilized, randomly picked PD-1 blocking antibodies instead of PD-L1 Fc did not have the same effect, suggesting the importance of the binding site.
The researchers introduced a FLAG tag in tandem in the N-terminal N-loop of PD-1 (FLAG-hPD-1) or replaced a part of the PD-1 stalk region with a FLAG tag (hPD-1 stalk FLAG) to study whether an anti-FLAG antibody guided to these regions of the PD-1 molecule could stimulate its immunosuppressive activity. In an assay with co-immobilized anti-FLAG, anti-CD3, and anti-CD28 antibodies, IL-2 production was downregulated in DO11.10 cells transfected with hPD-1 stalk FLAG, but not FLAG-hPD-1 or wild-type hPD-1, suggesting that the recognition of the stalk region may be important for the stimulation of the immunosuppressive activity of PD-1.
As a cell-based readout, Suzuki and Tajima et al. used the PD-1 knockout I-Ad-restricted T cell line DO11.10, which upon stimulation with OVA323-339-pulsed IIA1.6 B lymphoma cells produces IL-2. When DO11.10 cells transfected with wild-type hPD-1, FLAG-hPD-1, or hPD-1 stalk FLAG were used in this assay, IL-2 production was inhibited, due to interaction with PD-L1 on IIA1.6 cells. However, when the assay was set up with PD-L1- IIA1.6 cells, and anti-FLAG was added instead, no effect on IL-2 production from transfected DO11.10 cells was observed, leading the authors to speculate that Fc receptors on the antigen-presenting cell were needed to keep anti-FLAG, and later agonistic anti-PD-1 antibodies, at the immunological synapse and enable co-ligation of PD-1 and TCR.
Next, the researchers replaced the Fcγ receptor-deficient IIA1.6 cells in their assay with parental A20 cells and, as expected, observed decreased IL-2 production from hPD-1 stalk FLAG-expressing DO11.10 cells upon addition of anti-FLAG. Experiments with a critical mutation introduced in the intracellular domain (Y248F) confirmed that engagement of hPD-1 stalk FLAG with anti-FLAG triggered the same PD-1 signaling pathway as PD-L1. Further, co-ligation studies with a truncated murine version of PD-1 stalk FLAG lacking the N-loop and IgV region of PD-1 did not inhibit DO11.10 T cells, suggesting that these domains are also required for the immunosuppressive activity.
In order to be able to screen for agonistic anti-hPD-1 antibodies, Suzuki and Tajima et al. designed to two assays: a “blocking antibody assay” (hPD-1+ DO11.10 cells plus Fc-receptor-deficient hPD-L1+ IIA1.6 cells) and an “agonist antibody assay” (hPD-1+ DO11.10 cells plus mFcγRIIB+ hPD-L1- IIA1.6 cells). The researchers assessed a panel of 81 anti-hPD-1 antibodies with diverse binding epitopes for their capacity to bind to hPD-1. Further, they were able to classify ectopic-regions of each anti-hPD-1 antibody by assessing binding to hybrid PD-1 molecules for which eight different segments of the extracellular domain of hPD-1 were individually swapped with the corresponding mPD-1 segments. This way, Suzuki and Tajima et al. identified blocking anti-hPD-1 antibodies binding to the membrane-distal region of hPD-1 and agonistic antibodies with strong immunosuppressive activity recognizing the membrane-proximal extracellular region (MPER) of hPD-1.
Agonistic antibody clone HM266 (mouse IgG1) binding to segment #7, proximal to the stalk region of the PD-1 molecule, was selected for further characterization. HM66 did not interfere with binding of PD-L1 or PD-L2 to PD-1, and was additive with PD-L1. The intensity of immunosuppression was directly dependent on the levels of PD-1 on the T cell surface and levels of FcγRIIB on the antigen-presenting cell. Stimulation of PD-1 by HM266 led to downregulation of TCR signaling and prevention of ERK phosphorylation in DO11.10, similar to stimulation with PD-L1.
Moving on to the human system, Suzuki and Tajima et al. generated chimeric human IgG1 and IgG4 versions of HM266 and tested the antibodies in a coculture of human CD4+ T cells and THP-1 cells (a human monocytic cell line with APC properties) and monitored IFNγ production. The researchers observed some reduction in IFNγ, which was notably improved in coculture with THP-1 cells overexpressing hFcγRIIB. The immunosuppressive activity of HM266 could be further enhanced by the use of hIgG1 Fc variants with enhanced affinity to hFcγRIIB (HM266-hIgG1-X4). In a mixed lymphocyte reaction, HM266-hIgG1-X4 suppressed allogeneic T cell responses to primary B cells from healthy donors.
In a mouse model of acute graft-versus-host disease induced by transfer of spleen cells from C57BL/6 human PD-1 knock-in mice to B6D2F1 mice, HM266 (mIgG1) treatment reduced the expansion of H-2b+ H-2d- donor-derived T cells – many of which were hPD-1+ – in host mice, and prevented body weight loss. In a colitis model created by transfer of naive hPD-1 knock-in CD4+ T cells into RAG2-/- mice, treatment with HM266 reduced the expansion of CD4+ T cells in the lamina propria and the number of pathogenic effector cells (IFNγ- and/or IL-17-producing CD4+ T cells) in the lamina propria and mesenteric lymph nodes. Further signs for the prevention of colitis in this model after HM266 treatment were the avoidance of body weight loss and shortened colon length.
Finally, the researchers also showed an effect of agonistic anti-PD-1 on humoral immune responses. Treatment with HM266 reduced OVA-specific plasma antibody levels upon immunization of hPD-1 knock-in mice with 4-hydroxy-3-nitrophenyl acetyl-ovalbumin (NP-OVA) in alum. To rule out an effect of T cell depletion, treatment with HM255 (an IgG1 anti-PD-1 antibody with similar binding affinity to PD-1, but no agonistic activity) did not abrogate OVA-specific antibody production. These findings may become relevant for the pharmaceutical regulation of autoantibody production.
Besides its use in cancer immunotherapy, PD-1 is also an interesting drug target for the immunoregulation of a wide range of inflammatory disorders. Understanding how antibodies regulate PD-1 activity is important to translation applications of anti-PD-1 therapy. Suzuki and Tajima et al. showed that agonistic anti-PD-1 mAbs bind to the membrane-proximal region of PD-1 and engaged with Fc receptors on antigen-presenting cells to enable PD-1 crosslinking and transmission of the inhibitory signal. These findings may contribute to the development of agonistic anti-PD-1 antibodies as immunosuppressants for the treatment of various inflammatory diseases.
Write-up and image by Ute Burkhardt




