Surviving breast cancer is an incredible feat, but patients whose initial tumors are successfully cleared still face a high risk of recurrence, even years after initial treatment. Tumor recurrence can be attributed to the presence of dormant disseminated tumor cells (DTCs) that hide out in the body and can potentially seed new tumors later on. In recent work published in Cancer Cell, Goddard et al. investigated just how these sneaky cells evade the immune system, and found that it may just come down to a numbers game.
To begin, Goddard et al. established two models of DTCs. In the first model, parental 4T1 or 4TO7 mammary tumor cells, or the same cells engineered to express a dominant eGFP neoantigen and firefly luciferase, were transplanted into Balb/c mice. While tumors expressing the dominant neoantigens were spontaneously rejected by endogenous antigen-specific T cells, which were present in the spleens, lymph nodes, and bone marrow, eGFP+ DTCs remained in the bone marrow, evading antitumor immunity. This did not appear to be due to T cell exhaustion or dysfunction, as antigen-specific T cells remained active, functional, and capable of expanding and protecting mice upon rechallenge. In the second model, D2.0R mammary tumor cells expressing eGFP were intravenously administered to Balb/c mice. D2.0R cells persisted as solitary cells or small non-proliferative clusters within the lungs through day 240, despite the presence of antigen-specific T cells.
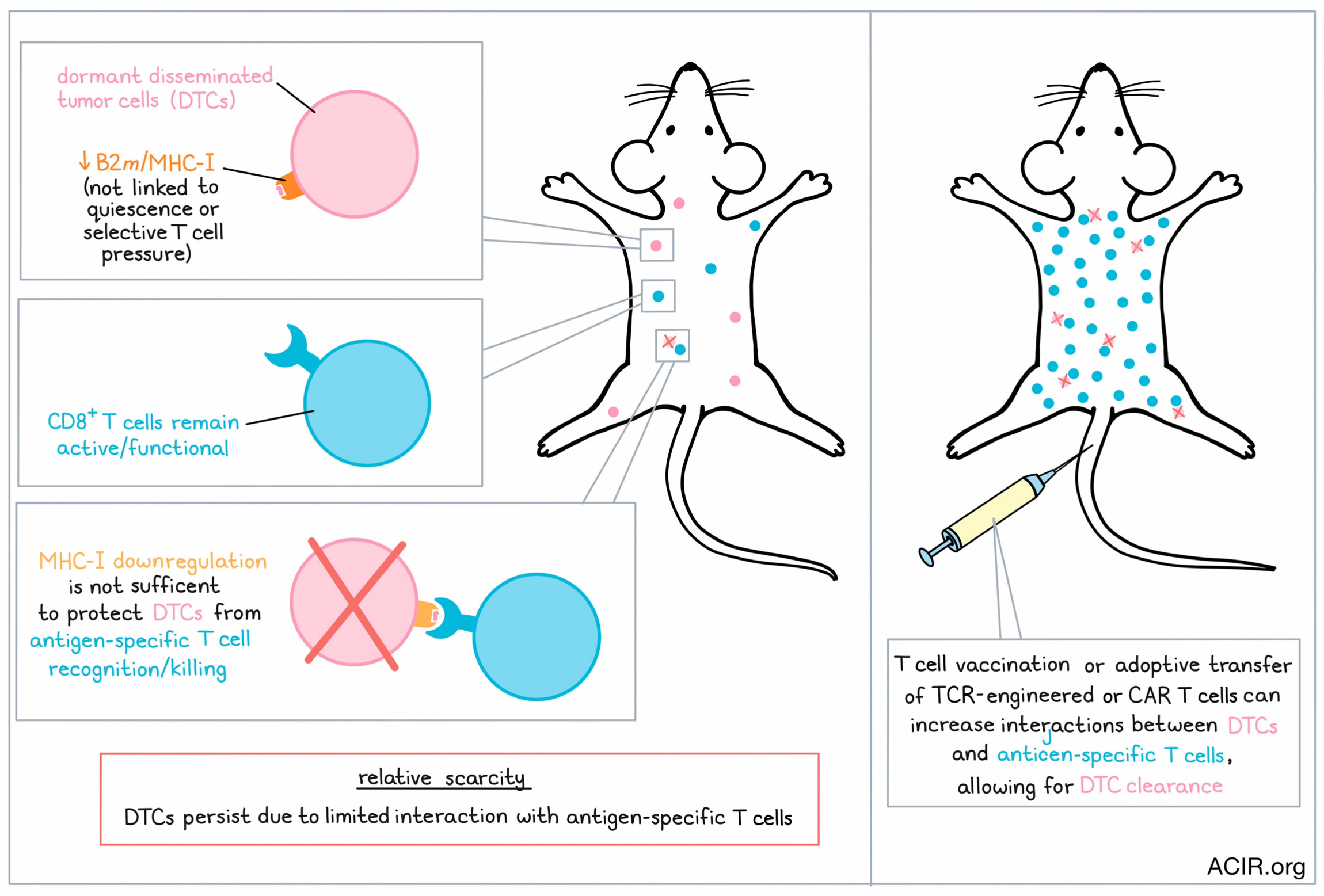
Comparing the D2.0R-eGFP model, which seeds dormant micrometastases, to another model, D2A1, which seeds proliferative metastases, the researchers noted downregulation of B2m, (a critical component of MHC-I that is essential for antigen presentation) over time in the D2.0R-eGFP cells. MHC-I was also downregulated at the protein level in these cells, suggesting a possible mechanism of immune evasion. Despite reduced MHC-I expression in D2.0R-eGFP DTCs, the levels of MHC-I expressed in proliferating and non-proliferating D2A1 cells were similar, suggesting that MHC-I downregulation was not linked to quiescence. It was also not due to selective pressure from the adaptive immune system, as similar patterns were observed in T cell-deficient athymic mice.
To determine whether the extent of the MHC-I downregulation would actually be sufficient for DTCs to evade antitumor T cells, the researchers developed microvascular niche (MVN) culture systems that, depending on the culture, could drive T4-2 breast cancer cells towards their typical proliferative state, or towards a quiescent state that mimicked the phenotype of MHC-I downregulation by quiescent DTCs observed in mice. Using these systems to co-culture T4-2 cells engineered to express NY-ESO-1 alongside HLA-A2/NY-ESO-1-specific TCR T cells, the researchers found that the T cells eliminated most of the target cells, regardless of whether they were proliferative or quiescent. Testing the same concept in vivo, the researchers transplanted mice with syngeneic D2.0R-eGFP cells expressing influenza virus hemaglutinin (HA), which can be recognized by adoptively transferred CL4 TCR CD8+ T cells. Again, despite reduced expression of MHC-I, the tumor cells could be recognized and significantly cleared, suggesting that the level of MHC-I downregulation by DTCs was not sufficient to escape detection by antigen-specific CD8+ T cells.
Goddard et al. hypothesized that CL4 TCR T had likely cleared the DTCs with higher MHC-I expression, leaving only those with lower MHC-I expression, but analysis showed that persistent DTCs maintained a full spectrum of MHC-I expression levels. Further, when the researchers treated mice harboring residual DTCs with IFNγ, upregulating MHC-I expression, DTCs still persisted in the presence of either endogenous T cells or adoptively transferred TCR T cells. These results further suggest that reduced MHC-I expression is not the driving mechanism behind DTC immune evasion.
Following observations that adoptive transfer of high volumes of antigen-specific T cells could be sufficient for DTC clearance, the researchers investigated the possibility that DTCs may evade immune detection simply due to limited interactions between the two relatively rare cell types. To study this mechanism of “relative scarcity”, the researchers used T cell-deficient AtN mice seeded with D2.0R-eGFP DTCs and transferred eGFP-specific JEDI T cells in log-fold dilutions. This showed a dose-dependent clearance of DTCs. As expected, the average distance between T cells and DCs also decreased with higher doses of T cells. Similarly, a T cell-based vaccination system that elicited expansion of endogenous CD8+ T cells against target antigens reduced dormant DTCs.
To test whether CAR T cells could clear DTCs, Goddard et al. used T4-2 breast cancer cells engineered to express a modified CD19 with no signal transduction capacity in coculture with CD19 CAR T cells. Here, the researchers saw a 93% reduction in both proliferating and quiescent target cells. For in vivo studies, the team used mice seeded with tCD19+ D2.0R cells and treated them with CD19 CAR T cells. This eliminated an average of 98% of DTCs in mouse lungs, outperforming the previous studies with tumor antigen-specific T cells. Similar results were observed using CAR T cells targeting the known breast cancer antigen HER2 in mice bearing HER2+ BT474 or HCC1569 human breast cancer lines, which can become proliferative or dormant depending on their location in the body. A mouse-optimized version of a trastuzumab-based CAR construct currently under clinical investigation was also effective at clearing DTCs.
Overall, these results show that while DTCs downregulate MHC-I, they primarily evade immune detection through “relative scarcity” – limited interactions between DTCs and the T cells that might recognize them. Immunotherapies that increase T cells specific for a known DTC antigen, like vaccination or the adoptive transfer of TCR-engineered or CAR T cells, can help to overcome relative scarcity, clearing DTCs that might potentiate tumor recurrence.
Write-up and image by Lauren Hitchings




