Colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC) have a low survival rate, low infiltration of tumor-specific tumor-infiltrating lymphocytes (TIL), infrequent neoantigen mutations, and checkpoint inhibitors are largely ineffective in PDAC and subtypes of CRC. Common mutations in the oncogenes driver KRAS (mKRAS) however can serve as immunotherapy targets. Pant et al. conducted a phase 1 trial of a vaccine strategy in these cancer types to overcome some of these challenges, the results were recently published in Nature Medicine.
ELI-002 2P is a 3-component lymph-node-targeted vaccine that comprises amphiphile (Amph)-modified G12D and G12R mKRAS long peptides, and Amph-modified TLR9 agonistic CpG-7909 DNA. The diacyl lipids in the amphiphile can bind to fatty-acid-binding pockets on albumin, leading to molecular “hitchhiking” of the vaccine components. This results in increased lymph node accumulation and delivery to APCs to optimize its effects.
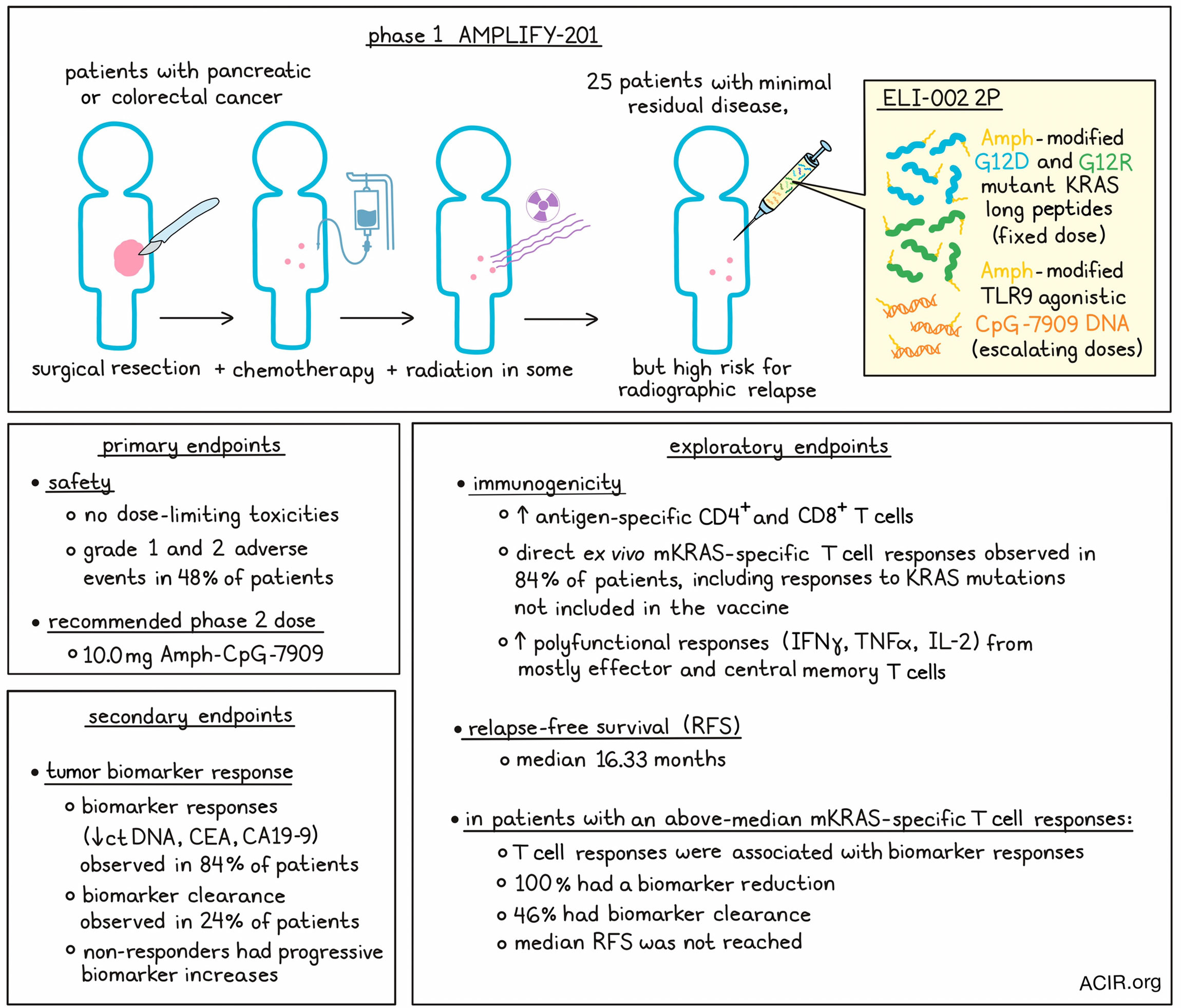
The researchers assessed the safety, efficacy, and immunogenicity of the vaccine, as well as established the recommended phase 2 dose (RP2D) in the AMPLIFY-201 phase 1 study. Patients had received definitive locoregional therapy (chemotherapy and surgery; 28% of patients also received radiation), and were positive for minimal residual disease (MRD) with a high risk for radiographic relapse. The relapse risk was based on circulating tumor DNA (ctDNA) or serum tumor antigen biomarkers carbohydrate antigen 19.9 (CA19-9) or carcinoembryonic antigen (CEA) positivity. The presence of tumor mKRAS mutations (G12D or G12R), as assessed by whole-exome sequencing, was also an enrollment criterion.
The trial enrolled 25 patients (PDAC n=20, CRC n=5) who all received a fixed dose of Amph-Peptides 2P, with escalating Amph-CpG-7909 doses in separate cohorts, using a modified 3+3 design. All patients could be assessed for safety, immunogenicity, and preliminary antitumor activity. Treatment was discontinued early in 13 patients due to disease progression. Drug-related adverse events were experienced by about half of the patients (48%), and were all Grade 1 or 2. The most observed events were fatigue, injection site reactions, and myalgia. There was one serious adverse event related to a biopsy taken for the study. The RP2D was determined to be 10 mg of Amph-CpG-7909, and this dose was safe and well tolerated.
To assess tumor response, the secondary endpoint of the study was a decrease from baseline in ctDNA and/or serum tumor antigen levels (CA19-9 or CEA). Most patients (84%) had a decline from baseline, which was ≥30% in 44% and ≥50% in 32% of patients, while 24% had complete biomarker clearance of ctDNA. Serum CEA or CA19-9 declines contributed to tumor biomarker response, but not clearance (which returned to normal levels in some patients). Declines were seen in both PDAC and CRC, and all patients with G12R and 80% of those with G12D mutations had biomarker decline. Responses did not differ depending on the time from prior surgery or chemotherapy. All patients who had undergone prior splenectomy responded to the vaccine, with 50% experiencing tumor biomarker clearance. Non-responders had progressive biomarker increases, and two patients had initial rising biomarker levels followed by a reduction to non-detectable levels.
An exploratory endpoint was the immunogenicity of the vaccine strategy. Peripheral blood mononuclear cells (PBMCs) were obtained and ex vivo stimulated for various mKRAS and WT antigens and directly measured by ELISPOT or intracellular staining for T cell responses. In one example patient, increased T cell immunity to the vaccine epitopes was detected after the prime immunization series, with 79% of T cells secreting IFNγ and 18% secreting granzyme B. Baseline responses to the epitopes were observed, but the vaccination greatly expanded the frequency of these pre-existing T cells. Responses to other mKRAS antigens not included in the vaccine (including other mutations at position 12) were also observed, but not to WT antigen. After vaccination, polyfunctional responses in both CD4+ and CD8+ T cells were observed (assessing IFNγ, TNFα, and IL-2), and most of these cells were of a central and effector memory phenotype. Immune responses were independent of known class I HLA restriction, suggesting significant cross-reactivity or the possibility of new HLA/epitope pairs.
When all 25 patients were assessed for immunogenicity, 84% had direct ex vivo T cell responses. At the highest two dose levels (5 and 10 mg Amph-CpG-7909), all patients had elevated mKRAS-specific T cell responses after vaccination, and 59% of patients had a specific response including both CD4+ and CD8+ T cells. Of the immune responders, 86% had T cell responses to ≥2 mKRAS antigens, and 52% induced T cell responses to all seven mKRAS antigens tested. In all patients, ex vivo responses were developed to 30/50 of the included vaccine epitopes. Of the immune responders, 67% had pre-existing mKRAS-specific T cells, but immune responses were also observed in the seven patients without detectable pre-existing mKRAS-specific T cells. In 6/7 patients whose T cell responses could be assessed longitudinally, maintenance of the higher T cell responses or further increases were detected after a boost. For 6 patients with progression, the tumor was biopsied, and the progression did not seem to have been mediated by poor T cell infiltration.
Other exploratory endpoints were recurrence-free survival (RFS) and overall survival (OS). The median OS and RFS were both 16.33 months. The researchers assessed the association between the induced expansion of mKRAS-specific T cell responses with tumor biomarker and RFS endpoints. Splitting T cell responses on the median, above-median responses correlated with biomarker-assessed clinical antitumor activity, and those with below-median responses more often had progression and no clearance. Among those with above-median responses, 100% had biomarker reduction, 46% had biomarker clearance, and the median RFS was not reached in this group.
These data suggest that ELI-002 2P vaccination can safely induce and increase mKRAS-specific T cell responses in patients previously treated for PDAC and CRC with a poor prognosis. Given that the induced expanded T cell responses correlated with decreases and even complete clearance of tumor biomarkers and longer RFS, this treatment may aid antitumor immune responses and has the potential to be combined with other immunotherapeutics to further improve long-term tumor control. ELI-002 is being further tested as a seven-peptide formulation in a phase 1 and randomized phase 2 study.
Write-up by Maartje Wouters, image by Lauren Hitchings.
Meet the researcher
This week, co-lead author Christopher Haqq answered our questions.

What was the most surprising finding of this study for you?
We were surprised and happy to see 84% of treated patients have T cell responses to our lymph node-targeted cancer vaccine ELI-002 2P. Since participants had previously received powerful chemotherapy regimens that can lower the number of immune cells present, it was great to see our data confirm that immunotherapy worked well here. It was also intriguing to see the correlation of high levels of T cells to reduced relapse in the study.
What is the outlook?
We are expanding the studies of lymph node targeting with a new formulation of ELI-002 that broadens the spectrum of KRAS mutations covered to include seven that are found in 25% of solid tumors. A randomized phase 2 study in pancreatic cancer has just started dosing, and there’s opportunity to expand to colon cancer and lung cancer studies as development progresses.
What was the coolest thing you’ve learned (about) recently outside of work?
I enjoy astrophotography as a hobby, and it’s been really cool to see the impact of artificial intelligence when I process images of deep space objects this year – the noise reduction makes my basic equipment shine!




