With the success of immune checkpoint blockade (ICB) therapies, the search is on for an understanding of the mechanisms and biomarkers to predict patient response. Lin et al. and Tang et al. tackled this problem head on by examining the role of PD-L1 expression on tumors versus host cells during ICB therapy, and separately came to the same surprising conclusion: host cell expression, and not tumor expression, of PD-L1 is essential for antitumor response during PD-L1/PD-1 blockade therapy.
To demonstrate that an intact host adaptive immune system is necessary for ICB efficacy, Lin et al. inoculated one of four cancer cell lines (MC38 colon cancer, ID8 ovarian cancer, B16-F10 melanoma, and LLC lung cancer) into mice with variable degrees of immunodeficiency: wild-type (WT), Rag-/- (no mature T, B cells), and NSG (no mature T, B, NK cells). Tang et al. performed similar experiments using Rag-/- mice with MC38 tumors. The researchers found that treatment of mice with anti-PD-L1 antibody reduced tumor growth and improved survival in WT mice with MC38, ID8, and B16-F10 tumors, and had no effect on LLC tumors. Meanwhile, PD-L1 blockade had no antitumor effect in NSG and Rag-/- mice in any tumor models, supporting the expected essential role of adaptive immunity for ICB efficacy.
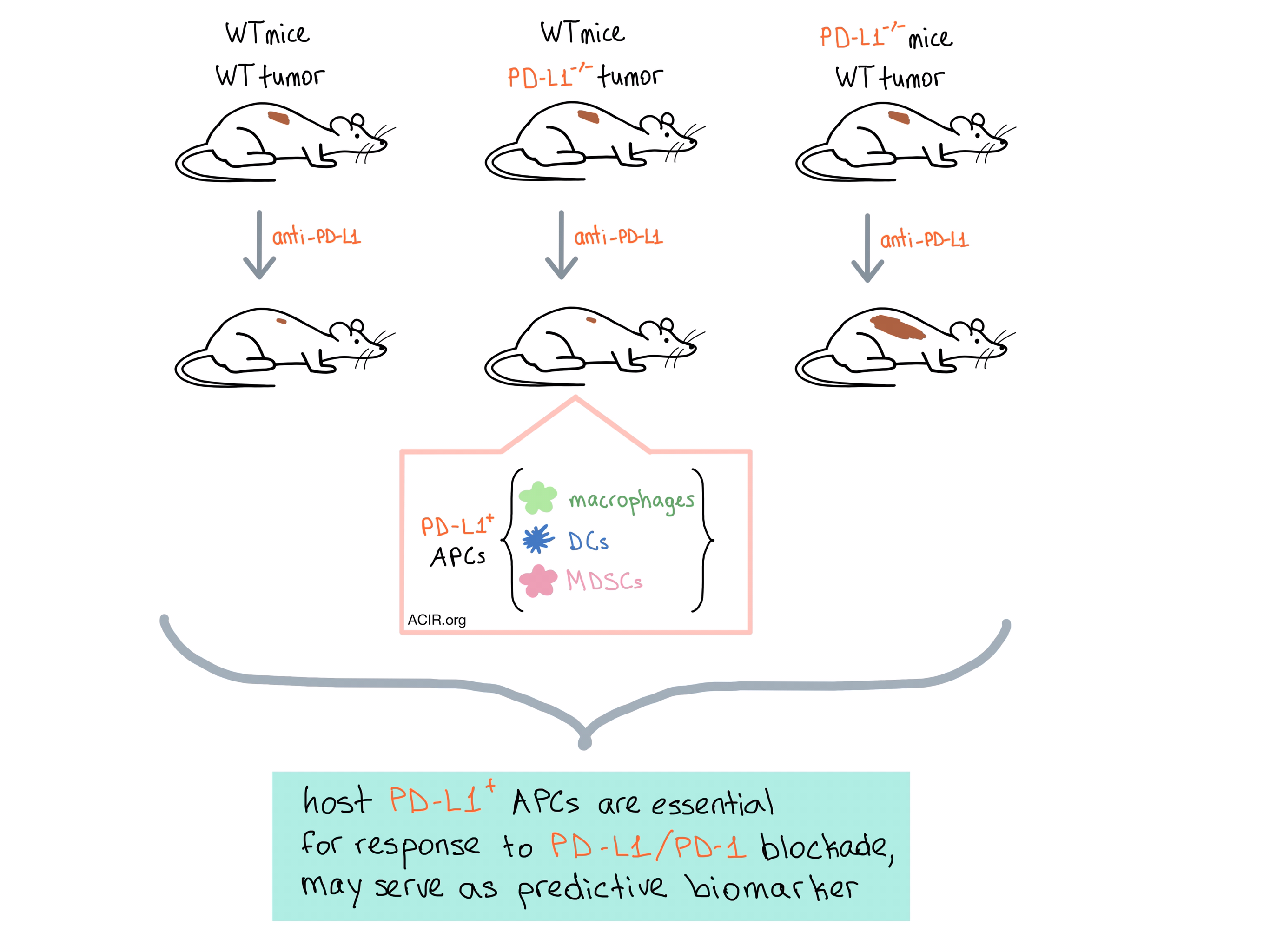
Next, the researchers wanted to elucidate the role of host PD-L1 and PD-1 in tumor immunity. Lin et al. inoculated PD-L1-/- and PD-1-/- mice with MC38, ID8, or B16-F10 tumors and found that the therapeutic effect of anti-PD-L1 or anti-PD-1 antibody treatment was abrogated in both mouse models. Tang et al. also found that PD-1-/- mice with MC38 tumors had no response to anti-PD-L1. These results suggest that host PD-L1 and PD-1 expression may be necessary for response to PD-L1 or PD-1 blockade.
To isolate the effect of tumor-expressed PD-L1 from host PD-L1, both teams utilized CRISPR-Cas9 to knock out PD-L1 in ID8 and B16-F10 (Lin et al.), MC38 (Lin et al. and Tang et al.), and A20 B lymphoma (Tang et al.) tumor cells and inoculated them into mice. Surprisingly, the PD-L1-/- tumors responded to anti-PD-L1 therapy as well as WT tumors, reducing tumor growth and increasing survival, indicating that it is indeed the host’s, and not the tumor’s expression of PD-L1 that is essential for anti-PD-L1 treatment efficacy.
So, if anti-PD-L1 treatment is not exerting its effect by acting directly on the tumor, what is it actually doing? Lin et al. examined the T cells within the tumor microenvironment (TME) and in tumor-draining lymph nodes (dLNs) in anti-PD-L1-treated mice, and found that CD4+ and CD8+ T cells were activated not only in the tumor but also in the dLNs of WT mice, but that no activation occurred in either venue in PD-L1-/- or PD-1-/- mice. Tang et al. found similar effects in the dLNs and, using an inhibitor of lymphocyte trafficking out of dLNs, concluded that efficacy depended significantly on T cell extravasation from dLNs. These data imply that PD-L1 is involved not only in the T cell effector phase within the TME, but also, more importantly, in the T cell priming phase within dLNs. This suggests that blocking PD-L1 signaling in the lymph nodes could contribute to tumor control.
After all these experiments, the question remained: which host cells are the target of anti-PD-L1 therapy? Using whole-body PET/CT with radiolabeled anti-PD-L1 antibody injected into tumor-bearing mice, Tang et al. found that the antibody targeted the tumor tissue at similar levels in both WT and PD-L1-/- tumors. Furthermore, using bone marrow chimeras, they were able to directly implicate bone marrow-derived cells as a key host cell target. Both groups then analyzed PD-L1 expression and function in host immune cell subsets in tumor-bearing mice and found high levels of PD-L1 expression on macrophages, myeloid-derived suppressor cells (MDSCs), and dendritic cells (DCs) in the tumors and dLNs, eventually concluding that tumor-associated antigen-presenting cells (APCs) are PD-L1+, regulate T cell function, and are the main targets of anti-PD-L1 therapy.
To find out if these results are relevant in human cancers, Lin et al. cultured human T cells from peripheral blood with DCs, macrophages, and radiated ovarian cancer cells, and found that DCs and macrophages, but not tumor cells, efficiently activated the T cells, and this effect was further enhanced by anti-PD-L1 treatment. The researchers then used multi-color immunofluorescence on tissue sections from patients with locally advanced and metastatic melanoma who received PD-1 and CTLA-4 checkpoint blockade and found a positive correlation between the percentage of PD-L1+ DCs and macrophages within the tumor and complete clinical response. Lin et al. also observed a positive correlation between APC PD-L1 expression and general clinical response to PD-1 blockade in patients with ovarian cancer. Overall, the data suggests that PD-L1+ APCs may correlate with clinical efficacy of PD-1 blockade in melanoma and ovarian cancer patients.
Although neither research group excludes a role for tumor-expressed PD-L1 in anti-PD-L1 antitumor response, they both conclude that it is the host PD-L1+ APCs that play an essential part in mediating response to PD-L1 and PD-1 blockade, and that these cells may be the biomarker that predicts patient response to ICB therapy.
by Anna Scherer




