Timing is everything, especially when it comes to combination immunotherapy. This was the conclusion reached by two separate research groups that evaluated the combination of agonist-OX40 and anti-PD-1 antibody therapies in different mouse models.
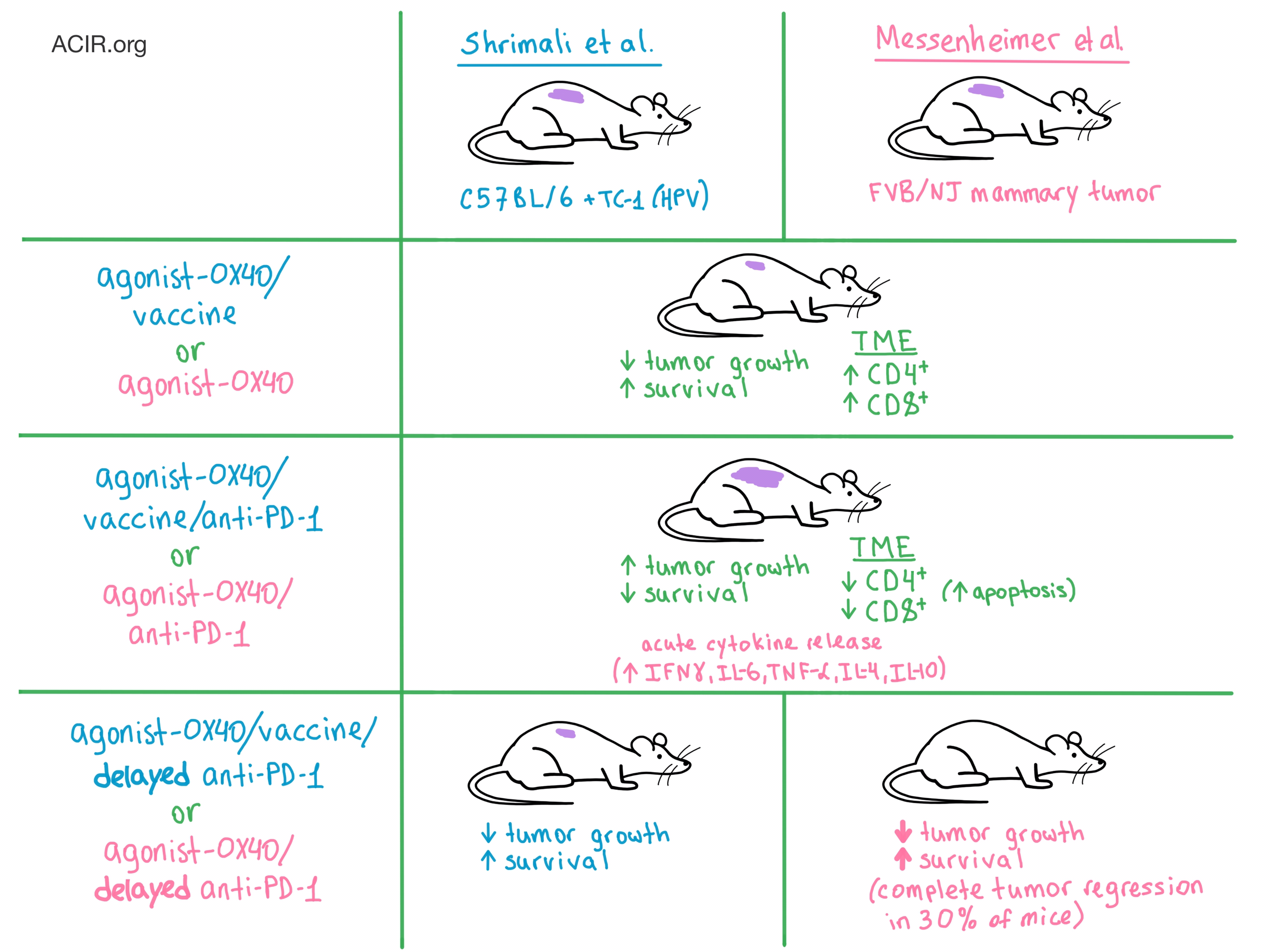
The researchers were inspired by the challenges of optimally combining immunotherapies in the clinical setting. To address this problem, Shrimali et al. utilized a syngeneic mouse model in which C57BL/6 mice were implanted with TC-1 cells (an HPV E6- and E7-driven tumor). This model requires a vaccine (an epitope from HPV protein E7) to create an effector immune response. Meanwhile, Messenheimer et al. orthotopically transplanted spontaneous tumors from FVB/NJ mice with mammary cancer into naive FVB/NJ mice. This mouse model does not utilize a vaccine, is refractory to PD-1 blockade, and was selected to represent the patients who do not respond to PD-1 blockade in the clinic.
OX40 is a costimulatory receptor that promotes T cell activation and expansion, enhanced effector function, immune memory generation, and inflammatory antitumor response. When Shrimali et al. administered agonist-OX40 in combination with vaccine, the rate of tumor growth was significantly reduced and mice experienced prolonged survival compared with untreated mice. Messenheimer et al. observed similar results with the administration of agonist-OX40 alone.
Intending to further improve the antitumor response, the researchers on both teams concomitantly added anti-PD-1 to the treatment. Surprisingly, both teams witnessed a significant reduction in tumor control and survival compared with agonist-OX40 or agonist-OX40/vaccine alone.
Next, the teams decided to delay the anti-PD-1 administration in order to allow the agonist-OX40 treatment to first boost T cell production. Messenheimer et al. observed that sequential dosing led to significant delay in tumor growth, as well as complete tumor regression in 30% of mice, compared with agonist-OX40 alone or concurrent combination therapy. They also witnessed a significant increase in survival compared with concurrent therapy. Reversing the order of therapy (anti-PD1 first followed by agonist-OX40) proved to be much less effective. Messenheimer et al. also confirmed these results in the poorly immunogenic 4T1 mammary tumor model. Meanwhile, Shrimali et al. observed that although delaying the addition of anti-PD-1 was not detrimental to the efficacy of the agonist-OX40/vaccine treatment, it did not provide any additional therapeutic benefit, either in tumor growth or survival.
To understand why the timing and order of administration of the two immunotherapy agents significantly influenced the therapeutic outcome, the researchers explored the effects of treatment on various T cell populations and secreted cytokines. Shrimali et al. noticed that the agonist-OX40/vaccine treatment increased the number of CD4+ and antigen-specific CD8+ T cells in the tumor, but the concomitant addition of anti-PD-1 significantly reduced the number of all tumor-infiltrating T cells, with the exception of CD4+ Tregs. Adding anti-PD-1 also led to significantly increased production of IFNγ, which resulted in enhanced CD8+ T cell proliferation and activation followed by increased apoptosis, both in the tumor and the spleen. High throughput immunosequencing demonstrated a decrease in T cell clonality after the addition of anti-PD-1 to agonist-OX40/vaccine, further suggesting apoptosis of antigen-specific T cells. In contrast, delaying the addition of anti-PD-1 did not alter the proliferation and apoptosis of CD8+ T cells compared to agonist-OX40/vaccine alone, which may help explain the distinct therapeutic results observed between concomitant and delayed administration of anti-PD-1.
Messenheimer et al. also observed a large increase in IFNγ production after concomitant combination therapy compared to either monotherapy and an increase in other cytokines, including IL-6, TNF-α, IL-4, and IL-10. Combined with certain physical symptoms (unkempt fur, lethargy), the researchers concluded that the animals experienced acute cytokine release. The researchers also saw that concurrent combination therapy increased T cell proliferation, but this effect was short-lived and did not correlate with improved therapeutic response due to rapid and increased apoptosis. In addition, concurrent combination treatment increased the frequency of exhausted TIM-3+CD8+ T cells in the tumor compared with agonist-OX40 alone. In contrast, sequential addition of anti-PD-1 did not lead to either abrogated T cell proliferation, increased T cell exhaustion, or spikes in cytokine production (indicating a safer, more effective profile). Additional depletion experiments demonstrated that both CD4+ and CD8+ T cells were required for optimal therapeutic effect: the former for immediate tumor control, and the latter for long-term memory response.
The results of these studies by Shrimali et al. and Messenheimer et al. exquisitely demonstrate the delicate “compensatory, regulatory, and homeostatic” balance that is maintained by the immune system, and underscore the importance of timing in the design of combination immunotherapy for cancer.
by Anna Scherer




