While a therapeutic human papilloma virus type 16 (HPV16) vaccine can effectively promote an immune response and subsequent durable regressions of HPV16-induced premalignant lesions, it has shown no clinical benefit in patients with late-stage HPV16-induced cervical cancer. To address the immunosuppressive environment that prevents the vaccine from inducing a strong immune response in such cancers, Melief and Welters et al. conducted a clinical trial that evaluated the safety, immunogenicity, and clinical benefit of a therapeutic HPV16 vaccine in combination with myeloid cell-depleting chemotherapy. The results were recently published in Science Translational Medicine.
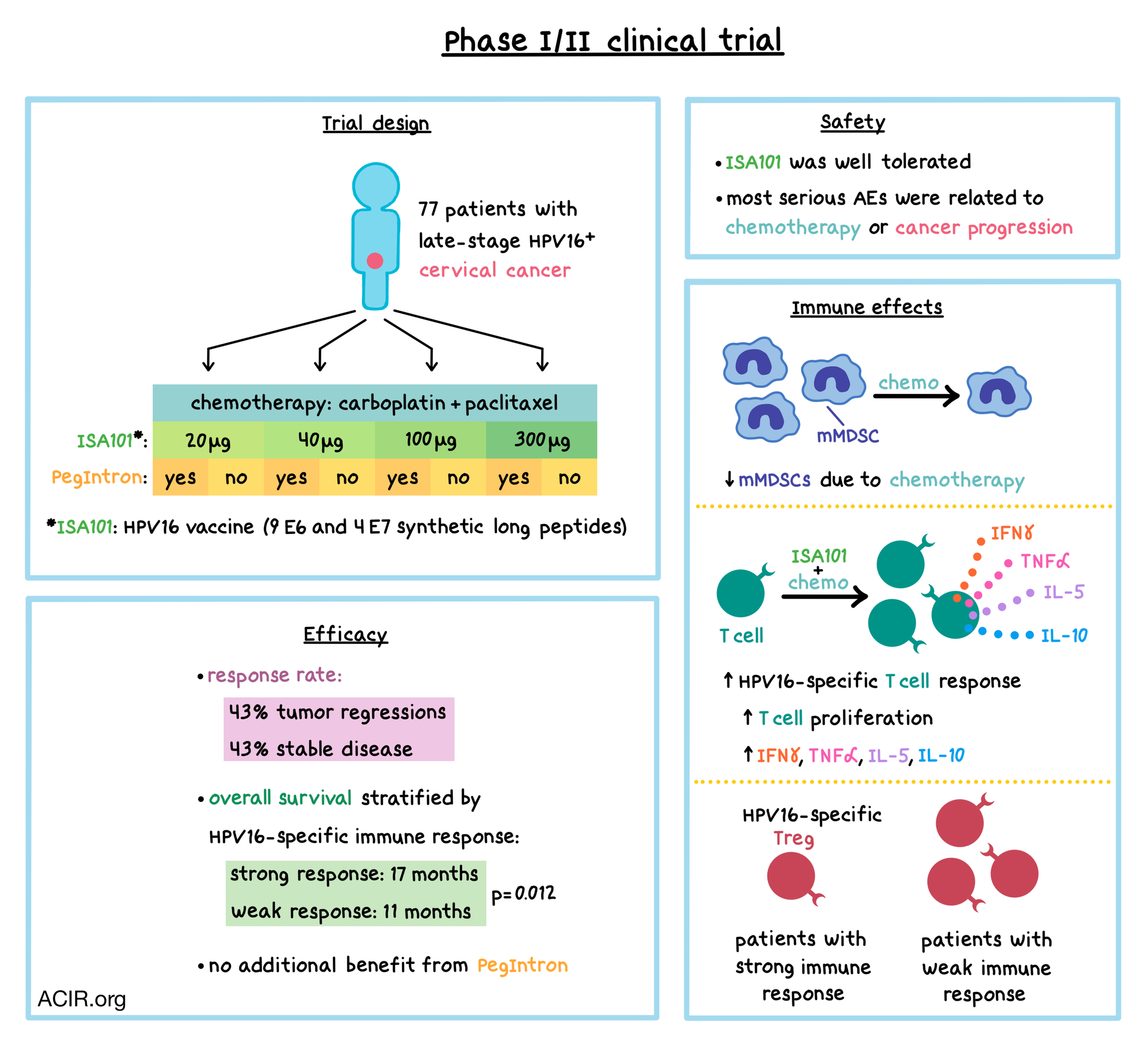
In this phase I/II dose escalation clinical trial, 77 patients with advanced (stage IIIb/IVa with involvement of lymph nodes beyond the renal vein), metastatic (stage IVb), or recurrent HPV16+ cervical cancer were treated with ISA101 – an HPV16-SLP (synthetic long peptide) therapeutic vaccine containing nine E6 and four E7 SLPs – in combination with carboplatin and paclitaxel. The carboplatin/paclitaxel chemotherapy combination was chosen because in preclinical studies, it reduced the number of immunosuppressive myeloid cells both locally and systemically. Patients were treated with six 3-week cycles of intravenous carboplatin and paclitaxel, as well as subcutaneous injections of ISA101 administered at week 2 of cycles 2, 3, and 4. The timing of initiation of ISA101 was based on previous determination that mononuclear myeloid cells reach their lowest levels at 2 weeks after the second cycle of chemotherapy.
Eight cohorts of patients were treated with different doses of ISA101 (20, 40, 100, or 300 μg per peptide; two cohorts per dose), with one cohort in each dose also receiving subcutaneous injections of pegylated type 1 IFN (PegIntron). PegIntron was added to the treatment regimen because IFNα promotes antigen (including long peptide) presentation by dendritic cells (DCs), upregulates costimulatory molecule expression and costimulatory molecule production by DCs, and, as shown in a previous clinical study, improves the vaccine-induced type 1 immune response.
ISA101 was generally safe and well tolerated. All patients experienced some adverse events (AEs), either due to chemotherapy, ISA101, IFNα, or a combination of any of the administered drugs. Most serious AEs were related to chemotherapy or progression of cancer. Among the 72 patients who experienced AEs that were possibly related to ISA101, 20 patients (28%) reported grade 3 or 4 AEs. Injection-related reactions were common and escalated with the dose, though most were grade 1 or 2. Allergic reactions to ISA101 were reported in 11 (15%) of the 72 patients with AE.
Immune cell analysis revealed that chemotherapy reduced the number of leukocytes circulating in the blood, while the number of lymphocytes was unchanged. Specifically, chemotherapy decreased the proportion of HLA-DR-CD14+ monocytic myeloid-derived suppressor cells (mMDSCs), but not HLA-DR+CD14+ myeloid cells, potentially indicating reduced immune suppression. Confirming this possibility, T cells collected from patients just prior to vaccination showed an increased memory response to a mixture of common microbial antigens, and a portion of patients developed a spontaneous, albeit low-frequency, T cell response against HPV16 E6 and/or E7 oncoproteins.
In all 64 evaluable patients, the ISA101 vaccine induced T cell responses specific for HPV16 E6/E7 peptides. The vaccine led to T cell proliferation and increased production of IFNγ, IL-5, IL-10, and TNFα. The strength of the vaccine-induced response did not vary across different doses, was not affected by preexisting HPV16 immunity, and was not impacted by pre-vaccination serum concentrations of immunosuppressive cytokines TGFβ, IL-4, or VEGF. ISA101 did not alter T cell recall response to unrelated antigens, indicating the HPV16-specific effect of the vaccine. The frequency of Tbet+ CD4+ and CD8+ T cells in peripheral blood correlated with the strength of HPV16 E6/E7-specific T cell responses, as indicated by the number of IFNγ-producing cells, and PegIntron treatment did not affect the frequency of Tbet+ T cells. After 3 or 4 vaccine doses, compared to patients with a strong response to the vaccine, patients with a weak response had significantly elevated percentages of circulating HPV16-specific Tregs, which are common in cervical cancers and likely contribute to suppression of the immune response.
The combination therapy led to tumor regressions in 43% of 62 evaluable patients, and another 43% experienced stable disease. Target lesions regressed in 29 of 59 patients who had measurable target lesions. PegIntron did not result in any additional clinical benefit in this trial. Different ISA101 doses did not affect tumor regressions or patient survival. When stratified by vaccine-induced HPV16-specific immune response, patients with a strong immune response had a longer median overall survival (OS) of 17 months (with a flat tail on the survival curve), compared to 11 months in patients with a weak immune response. The OS was not influenced by the preexisting HPV16-specific T cell response, and was not related to general T cell immune status, as reactivity to unrelated microbial antigens did not stratify survival.
Overall, this clinical trial demonstrates that carboplatin and paclitaxel chemotherapy selectively depletes immunosuppressive mMDSCs and allows an HPV16-targeted vaccine to induce an effective T cell immune response against late-stage, HPV16+ cervical cancer, leading to tumor regressions and prolonged survival.
by Anna Scherer
Meet the researcher
This week, Kees (Cornelis) Melief and Sjoerd van der Burg answered our questions.

What prompted you to tackle this research question?
KM and SVDB: The ISA101 vaccine was successfully used to (partially) eradicate HPV-induced premalignant lesions of the vulva in about 50% of the patients (See Kenter & Welters et al. NEJM 2009; van Poelgeest & Welters et al. Clin Cancer Res 2016), however, when we tested this in patients with advanced or a first recurrence of HPV-induced cervical carcinoma, no evidence of clinical efficacy was seen and a lower reaction of the immune system to the vaccine was observed (van Poelgeest & Welters et al. J Transl Med. 2013). To us this indicated a potential systemic and local immune suppression in patients with cervical cancer. In 2016 we showed (Welters, van der Sluis, van Meir et al. Science Transl Med 2016) that progressive cancer acts on the leukocytes as we found more immunosuppressive myeloid cells in the blood of these patients as well as in an animal model of HPV-induced cancers. We also showed that after two cycles of the standard carboplatin/paclitaxel chemotherapy this was altered in that the percentages of different myeloid cells in patients returned to their normal levels and immune suppression was less. We then tested if we could get better vaccine-induced immune responses if we would vaccinate patients after these two cycles of chemotherapy, and indeed this was the case; strong T cell responses to the vaccine were associated with prolonged overall survival.
What was the most surprising finding of this study for you?
KM and SVDB: There were two surprising findings. Over the years (from 2004 onwards) we had studied the spontaneous (disease-induced) immune response to HPV in patients with cervical cancer and always found this to be non-existent or very weak. In the current study, we also found that baseline responses were almost non-detectable. However, the chemotherapy-mediated alleviation of immune suppression revealed that about one-third of the patients had mounted an immune response while the remainder stayed negative. This showed that most of the patients with cancer in fact have failed to develop immunity to HPV after first contact in contrast to healthy people who have cleared the virus. The second surprise was the flat-tail at the end of the survival curve in patients with a strong immune response after vaccination. This is similar to what scientists noted when patients with melanoma were treated with ipilimumab, and, as we know, boosted a whole new era in the treatment of cancer with immunotherapy.
What was the coolest thing you’ve learned (about) recently outside of work?
SVDB: That the Dutch government takes guidance from well-seasoned scientists to handle the coronavirus crisis instead of following emotional or popular outbursts.
KM: That doctors from Cuba, endangering their own lives, travel to Italy, where more than 60 doctors have died, to assist in the crisis there.




