Having previously shown that ICOS stimulation via an ICOSL-transduced B16F10 cellular vaccine (IVAX) works synergistically with CTLA-4 immune checkpoint blockade (ICB) in various murine tumor models, Sharma et al. set out to explore the mechanism of action of this synergy. In a recent publication in the Journal of Experimental Medicine, they described their observations in the remodeling of the tumor microenvironment (TME) in response to this treatment.
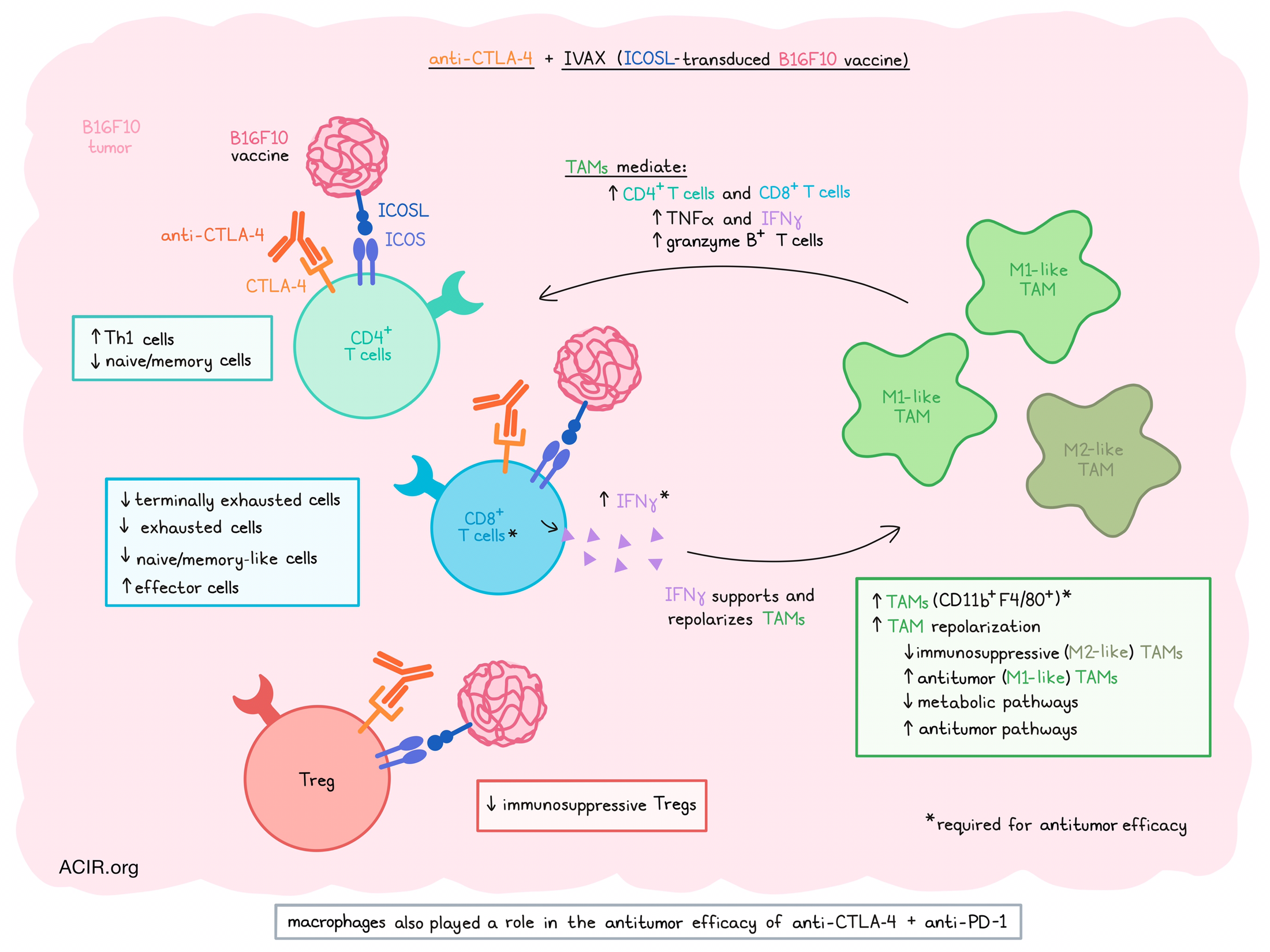
Treatment with CTLA-4 and IVAX of mice bearing B16F10 tumors resulted in increased infiltration of CD11b+F4/80+ tumor-associated macrophages (TAMs), and depletion of macrophages using clodronate liposomes confirmed that macrophages play a vital role in the antitumor immune responses generated by this combination therapy. Additionally, in vitro experiments showed that TAMs isolated from tumors receiving the combination treatment group were less immunosuppressive, resulting in more IFNγ production by CD8+ T cells. TAMs also had lower expression of CD206.
To further assess the role of ICOS signaling in the effects of the combination therapy, the researchers used scRNAseq and mass cytometry (CyTOF) to profile the tumoral immune populations after treatment. Mice with B16F10 tumors were treated with the combination of CTLA-4 ICB and IVAX or a vaccine comprising irradiated ICOSL-negative B16F10 cells (Vac). Tumors were collected and CD45+ cells were sorted for analysis. scRNAseq analysis resulted in 15 clusters of immune cell populations, including five monocyte/macrophage clusters, five T cell clusters, and one cluster each of classical DCs, plasmacytoid DCs, NK cells, neutrophils, and B cells. The combination treatment was found to modulate several of the cell populations, more than anti-CTLA-4 alone.
Reclustering the T cell subsets to gain more insight into the changes in this compartment revealed that a Treg cluster decreased, Th1 CD4+ T cell subsets increased, and a cluster of naive/memory CD4+ T cells decreased in the combination treatment group. Out of the CD8+ T cell clusters, three clusters, which were annotated as terminally exhausted, exhausted, and naive/memory-like CD8 clusters, decreased after treatment, two effector clusters increased in frequency, and a cluster of effector memory cells did not change in frequency.
CyTOF analysis into the effects of combination therapy on protein expression on various immune cell subsets revealed 19 T cell clusters. A Treg cluster characterized as a more immunosuppressive subtype decreased in the combination therapy group, as compared to anti-CTLA-4 monotherapy. Other CD4+ clusters that were considered Th1 cells were slightly increased in the combination therapy group. Among the CD8+ populations, an increase in effector cells was observed, while terminally exhausted cells decreased in frequency, as compared to anti-CTLA-4 monotherapy.
Moving to myeloid cells in the TME, the researchers reclustered the CD45+CD3- populations as determined by scRNAseq. This resulted in 15 clusters, including 8 monocyte/macrophage clusters. Four of these decreased in the combination therapy group, while one cluster increased. By assessing the expression of classical M1- and M2-type macrophage markers, it was established that the four clusters that were downregulated expressed genes associated with immunosuppressive TAM subsets. In contrast, the upregulated cluster had low or no expression of these genes and showed an antitumor phenotype. Gene analysis showed that antitumor pathways were increased after combination therapy, while most metabolic pathways were reduced in these macrophages.
Assessment of the macrophage populations by high-parameter CyTOF revealed 18 clusters among CD11b+CD3- immune cells isolated from the tumors, including 14 monocyte/macrophage clusters. Among these, four clusters decreased compared to CTLA-4 monotherapy; these clusters expressed CD206, CD204, LAP-TGFβ, and CX3CR1, suggesting an immunosuppressive phenotype. On the other hand, two clusters that increased after combination therapy did not express markers associated with suppressive macrophages. Altogether, these data suggest remodeling of the TME from one containing more immunosuppressive TAMs to one containing more non-suppressive TAMs.
Further assessing the role of TAMs, Sharma et al. depleted macrophages and studied the effects on T cell subsets in response to the combination therapy. The increase in effector CD8+ and CD4+ T populations in response to combination therapy was significantly reduced in mice treated with clodronate to deplete macrophages. The density of Tregs was unaffected; therefore, there was a substantial decrease in the CD8+ T cell-to-Treg ratio in clodronate-treated mice. Delaying clodronate treatment to eliminate the effects of clodronate on DC priming of T cells abrogated, but did not eliminate the CD8+ T cell reduction, indicating that cells beyond DCs were involved. Furthermore, combination treatment increased secretion of the proinflammatory cytokines TNFα and IFNγ by T cells, an effect almost entirely revoked when macrophages were depleted, resulting in cytokine levels comparable to those seen in tumors treated with anti-CTLA-4 monotherapy. Granzyme B-producing CD8+ T cells also decreased in the macrophage-depleted mice.
As IFNγ is a known driver of the M1 activation of macrophages, the researchers hypothesized that this cytokine may play an essential role in the macrophage and T cell remodeling observed in the TME of combination-treated mice. Treatment with an IFNγ-depleting antibody resulted in similar effects as clodronate treatment, reducing the number of TAMs and those expressing MHC-II, confirming the role of IFNγ.
Finally, the researchers hypothesized that these mechanisms might also be behind the efficacy of other combination therapy strategies. They investigated the effects of CTLA-4 and PD-1 ICB in the B16F10 model and how macrophage depletion by clodronate impacted the effects. Dual ICB resulted in the rejection of most tumors, while simultaneous macrophage depletion resulted in a reduction in tumor growth control and reduced survival.
Therefore, these findings suggest a positive feedback loop between T cells and TAMs in the TME in response to IVAX and anti-CTLA-4 therapy. In this loop, activated T cells produce IFNγ in response to treatment, which repolarizes TAMs to a more antitumor M1-like phenotype that, in turn, stimulates T cells further, resulting in a remodeling of the TME. These results suggest that anti-CTLA-4 treatment efficacy might be improved by ICOS agonism, and that caution should be taken when it comes to macrophage depletion in combination strategies with ICB, as positive effects of M1-like TAMs might be required for therapy efficacy.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, first author Naveen Sharma answered our questions.

What was the most surprising finding of this study?
The most unexpected finding in this study is that macrophage depletion decreased the efficacy of potent combination therapies, but not of single antibody therapy agents. Our data suggests that the effect could be accounted for by the increased IFNγ production by potent combination therapies, causing a positive feedback loop between T cells and TAMs, whereby the IFNγ produced by T cells polarize TAMs into the M1-like phenotype, hence leading to reprogramming of the tumor microenvironment to promote T cell infiltration and immune function. Our study further suggests that TAMs can play an antitumor role in tumor immunity, not just an immunosuppressive role.
What is the outlook?
Our study provides important insights into the mechanisms of combination immunotherapies. Developing effective combination therapeutic strategies requires a detailed understanding of the complex interactions between myeloid cells and T cells in the tumor microenvironment. Despite setbacks in recent clinical trials using anti-ICOS antibodies, our study further strengthens the case that combining an ICOS agonist with CTLA-4 instead of an anti-PD-1 blockade would be a wise strategy. It is also crucial to conduct extensive studies to define anti-ICOS antibodies' true agonistic/antagonistic nature, as their actual properties may differ from initial assumptions. Moreover, our study further highlights that future investigations are needed to fully understand the heterogeneity of myeloid populations and their functional relevance in antitumor immunity.
What was the coolest thing you’ve learned (about) recently outside of work?
Photography is the coolest thing I’ve learned recently, and it's been enjoyable. Before this, I had only a rudimentary understanding of photography. However, I took the plunge, invested in a quality camera, and enrolled in a short online course. As someone who enjoys hiking and observing wildlife, this newfound hobby seamlessly integrates into my outdoor adventures. While refining my photography skills, I’ve noticed a significant improvement in the quality of my images compared to those captured with a smartphone.




