Motivated by the limited response to immunotherapy among prostate cancer patients, Jachetti et al. explored the potential immunosuppressive role of mast cells in a paper recently published in Cancer Immunology Research. Mast cells are innate immune cells that promote angiogenesis and tumorigenesis, and they are known to promote the development of adenocarcinoma in the prostate.
For this study, the researchers chose the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model, which resembles human pathology. In TRAMP mice, progression of prostate cancer is driven by the transgenic expression of the oncogenic SV40 Large T antigen (Tag); because Tag in this system is a self-antigen, mice are peripherally tolerant despite tumor growth. Jachetti et al. first crossed TRAMP mice with mast cell-deficient KitWsh mice to determine whether mast cell deficiency would lead to reduced adenocarcinoma formation. They found that the KitWsh-TRAMP mice had significantly reduced adenocarcinoma growth compared with TRAMP mice. (Interestingly, the incidence of prostate neuroendocrine tumors increased. This suggests that mast cells promoted adenocarcinoma growth, but reduced the growth of aggressive neuroendocrine tumors. Mice with neuroendocrine tumors were not analyzed further in this study.) In addition, the team confirmed that CD8+ T cells were tolerant of the Tag epitope in TRAMP mice, but mounted a cytotoxic response to the same epitope in KitWsh-TRAMP mice both peripherally and within the tumor microenvironment, suggesting that mast cells play a role in CD8+ T cell inhibition.
To confirm these results, the researchers added bone marrow-derived mast cells (BMMCs) to KitWsh-TRAMP mice. The addition of BMMCs reduced CD8+ T cell activity to the levels found in TRAMP mice and significantly increased the incidence of adenocarcinoma. The link between T cell function and tumor growth was confirmed by T cell depletion experiments. Overall, these experiments demonstrate that mast cells inhibit CD8+ T cell activity and promote adenocarcinoma growth in TRAMP mice.
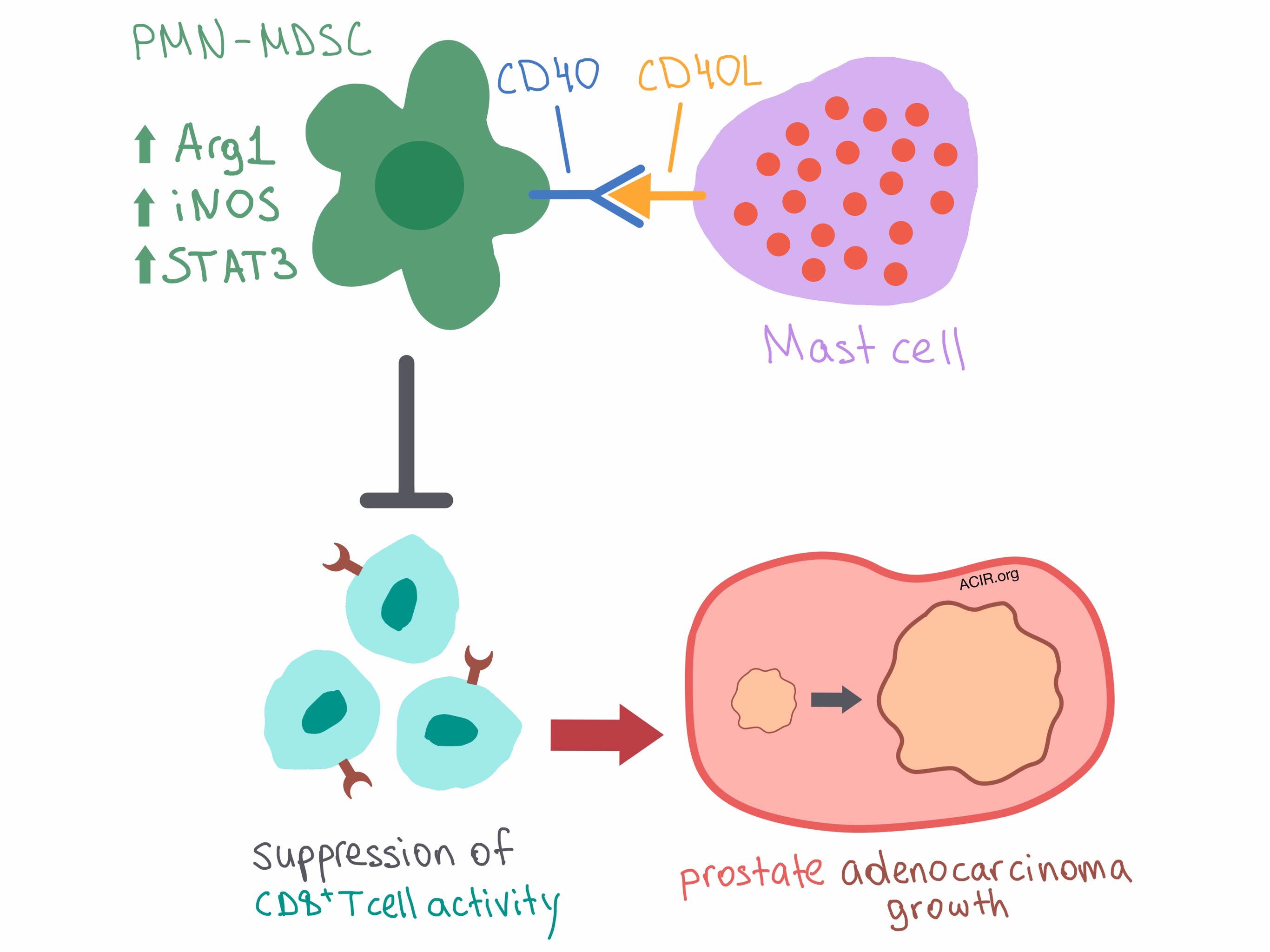
The researchers set out to understand the mechanism behind mast cell influence on CD8+ T cell activity. They demonstrated that mast cells did not affect the overall CD8+ T cell frequency in the spleen or CD8+ and CD4+ T cell infiltration into the prostate, ruling out non-specific T cell expansion. In fact, mast cells did not directly interact with CD8+ T cells in the spleen at all. Instead, the researchers found that mast cells colocalized with polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) in the spleen, where they communicated via CD40/CD40L binding. Ligation of CD40 on PMN-MDSCs triggered upregulation of Arg1, iNOS, and STAT3, which enhanced suppressive function. Thus, mast cells interact with PMN-MDSCs via CD40 to suppress CD8+ T cells, leading to cancer immune escape and adenocarcinoma development. Hinting at an additional level of complexity in the system, Jachetti et al. posited that additional, as yet unidentified, mechanisms involving mast cells and CD40L signaling may be involved in adenocarcinoma growth.
With clinical relevance in mind, the researchers analyzed human prostate cancer samples and found colocalization of mast cells and myeloid cells. Gene expression analysis indicated that high expression of mast cell/PMN-MDSC gene signatures correlated with biochemical relapse and reduced survival in prostate cancer patients. Overall, the results of this study suggest that CD40/CD40L signaling may be involved in immunosuppression in human prostate cancer, and the potential for enhancing immunosuppression may need to be addressed in future immunotherapy treatment designs utilizing CD40 agonism.
by Anna Scherer




