CAR T cell treatment (CAR-T) can be effective in hematological cancers, but is frequently associated with cytokine release syndrome (CRS), a severe adverse event. IFNγ has been implicated as a driving factor in the development of CRS, but is also considered essential for the efficacy of the treatment. Bailey et al. investigated how IFNγ blockade, using antibodies or deletion of IFNγ in CAR-T, affected treatment efficacy and whether it could prevent CRS. Their results were recently published in Blood Cancer Discovery.
The researchers first assessed whether the loss of IFNγ affected CAR-T tumor cell lysis. When IFNγ was blocked using an antibody in vitro, no differences were detected in granzyme B expression in response to exposure to CD19+ tumor cells, and the CAR-T could effectively lyse various cell lines. To test the in vivo response, NSG mice were injected intravenously with JeKo-1 lymphoma cells and then treated with the anti-CD19 BBζ CAR-T with or without the IFNγ-blocking antibody. The blockade resulted in reduced IFNγ serum levels, but mice could still clear the lymphoma. The CAR-T had similar engraftment levels in the blood, and overall survival was similar. In the more aggressive Nalm6 leukemia model, persistence and efficacy were also unaffected. Surprisingly, mice receiving IFNγ blockade had improved survival over mice that did not receive this treatment.
The authors developed anti-CD19 CAR-T (with a 4-1BB [BBζ] costimulatory domain) and used CRISPR/Cas9 guides to generate knockouts for IFNγ (IFNγKO). The specific deletion of IFNγ did not affect the subsets of CAR-T that were created. In both CAR-T and IFNγKO CAR-T products, CD4/CD8 ratios were similar and most of the cells were of a naive phenotype (CD62L+CD45R0-). Between the products, there were no significant differences in memory subsets or differences in cells expressing type 1 (CXCR3+CCR4-CCR6-) or type 2 (CCR4+CCR6-) chemokine receptors.
In the in vitro co-culture system, the IFNγKO CAR-T showed reduced IFNγ production compared to CAR-T, but maintained granzyme B expression and could lyse tumor cells. In mouse models, serum levels of IFNγ were lowered, but the IFNγKO CAR-T exhibited similar antitumor activity and engraftment to control CAR-T, and mice experienced similar survival. The researchers then assessed whether IFNγ was necessary for antitumor activity when CARs with a CD28 costimulatory domain (28ζ) were used, and again, IFNγ loss had no impact on the 28ζ CAR-T’s cytotoxic in vitro effects or in vivo behavior.
To establish whether this surprising phenomenon – in which IFNγ blockade does not affect CAR-T antitumor function – was restricted to CD19-expressing tumors alone, the authors similarly assessed B-cell maturation antigen (BCMA)-directed CARs. Again, a lack of IFNγ did not affect in vitro cytotoxicity. To assess the response to solid tumors, anti-mesothelin CAR-T were co-cultured with the pancreatic tumor cell lines BxPC-3 and Capan-2. Here, IFNγKO resulted in moderate resistance to the therapy. Antibody blockade of IFNγ also reduced the cytotoxic activity of anti-EGFR CAR-T against EGFR+ U87 glioblastoma cells. Therefore, IFNγ may be more important for the efficacy of CAR-T towards solid tumors than it is for hematologic cancers.
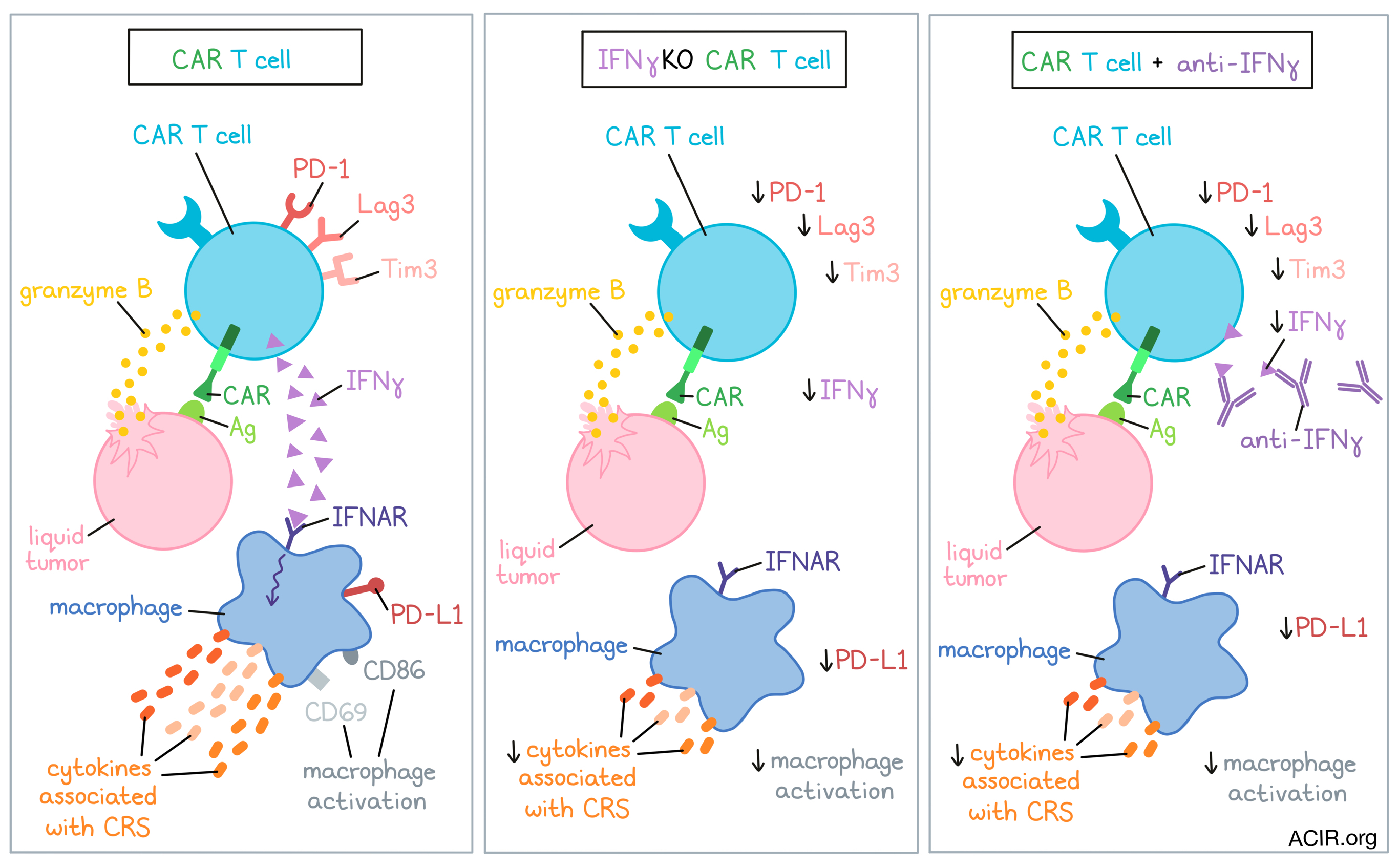
Because innate immune cells, such as macrophages, can amplify IFNγ signaling and may affect checkpoint molecule expression, the researchers assessed the impact of IFNγ loss on target-stimulated CAR-T phenotype. The CAR-T were co-cultured with Nalm6 cells with or without donor-matched macrophages derived from GM-CSF-activated monocytes. In the presence of macrophages, the IFNγKO BBζ CAR-T exhibited comparable proliferation, but lower expression of LAG3, PD-1, and TIM3, particularly in the CD4+ subset. A known feature of 28ζ-based CAR-T cells is a higher antigen sensitivity and cytotoxic activity than the BBζ CAR-T, but that comes with an increase in inhibitory molecules and reduced persistence. In this model, the IFNγKO 28ζ CAR-T had greater expansion than control CAR-T, and decreased upregulation of PD-1, TIM3, and LAG3, regardless of whether macrophages were present. Therefore, IFNγ may restrict the expansion of these CAR-T and increase their checkpoint molecule expression.
Next, Bailey et al. determined whether loss of IFNγ could prevent CAR-T-mediated macrophage activation, which may result in CRS. The researchers first established a profile of cytokines in serum samples collected from patients who had developed cytokine-related toxicities shortly after receiving anti-CD19 CAR-T products. These serum samples had high expression of cytokines, including IFNγ, IL-6, and IL-10.
The researchers then used healthy donor samples to show that macrophages derived from monocytes differentiated with GM-CSF best mimicked this patient cytokine profile in vitro. Macrophages were either added to CAR-T and Nalm6 tumor co-cultures, or were exposed to supernatants from CAR-T and tumor co-cultures. Both culture systems activated macrophages to produce the various CRS-related cytokines.
When the IFNγKO CAR-T were used in this model or when IFNγ was blocked with an antibody, there was a reduced production of cytokines and fewer macrophages were activated, as shown by reduced surface expression of activation markers CD86/CD69, reduced downstream IFNγ receptor signaling, and lower PD-L1 expression.
The researchers also developed a hybrid in vivo/in vitro model to assess macrophage activation. Serum from mice with Nalm6 tumors treated with CAR-T or IFNγKO CAR-T was added to donor-matched macrophages in vitro. The macrophages exposed to the serum of IFNγKO CAR-T-treated mice produced fewer cytokines, were less activated, and had lower PD-L1 expression.
Finally, Bailey et al. used their model system to assess how IFNγ targeting compared to current strategies used in the clinic to treat CRS. They did so by adding antibodies against IFNγ, IL-6R, or IL-1Ra to the contact-independent in vitro model. Anti-IFNγ treatment was more effective in reducing cytokine levels than the other two treatments. These results were confirmed with other specific macrophage populations (M0, M1, M2) and with undifferentiated monocytes. Transcriptional profiling of the macrophages also revealed that those treated with anti-IFNγ upregulated costimulatory genes and downregulated genes encoding PD-L2 and TIM3 – features that may improve CAR-T efficacy.
These data suggest that blockade of IFNy signaling does not affect CAR-T function against hematologic cancers, but might reduce the risk of CRS. To test this in the clinic, IFNγ-knockout in CAR-T might be a better approach than using an IFNγ-targeting antibody, as this latter treatment would also impact other immune cells. Finally, further research into the difference in effects found with solid tumors is of interest to better understand the mechanism of action.
Write-up by Maartje Wouters, image by Lauren Hitchings
Meet the researcher
This week, first author Stefanie Bailey answered our questions.

What prompted you to tackle this research question?
Chimeric antigen receptor T cell (CAR-T) therapy has yielded impressive outcomes in patients with blood cancers, but often triggers cell-mediated clinical toxicities that not only compromise the health of patients, but also the financial burden, as they can extend the length of hospitalization. Current cytokine antagonists in the clinic have not been successful at completely mitigating these adverse events, even when given prophylactically, likely due to targeting factors downstream of inflammation. One biomarker of cytokine release syndrome (CRS) is interferon gamma (IFNγ), a T cell-mediated cytokine that is a potent activator of innate immune cells. Inspired by the strong correlation between IFNγ and CRS in the clinic, we identified the IFNγ pathway as a potential target. Since this approach is targeting CAR-T directly, instead of downstream macrophage function, it's possible that clinical toxicities can be stopped before they begin. Reducing the potential toxicity of CAR-T therapy could make this life-saving approach more accessible to patients.
What was the most surprising finding of this study for you?
Although we were hopeful that IFNγ blockade would mitigate cell-mediated toxicities without compromising CAR-T function, there's a reason this approach has not been attempted in the past. IFNγ has long been associated with T cell effector function, and is used as the gold standard potency assay for CAR T cells. Recent work from our lab, and others, has shown that IFNγ is crucial for CAR T cells to effectively clear solid tumors. Additionally, IFNγ has been reported to be critical for immune checkpoint inhibitors. Given these findings, it's always been assumed that IFNγ is indispensable for T cell-based therapy. However, our surprising findings challenge that dogma and suggest that CAR-T efficacy and toxicity are not synonymous and can be separated to improve clinical responses.
What was the coolest thing you’ve learned (about) recently outside of work?
Being a postdoc and parent to two in a global pandemic has increased stress and anxiety to a whole new level. Before graduate school, painting was my favorite form of therapy, but that became less of a priority throughout the years. In a quest for some quiet time, we recently re-discovered the simple, yet magical therapy of coloring with our 3-year-old. We now have coloring nights a couple times a week as a way to recover from the chaos of life. If you're burnt out on science, parenting, or just life in general, I 10/10 recommend finding a coloring book and letting go for a while. Paw Patrol recommended, but to each their own. Love your family, work hard, enjoy the science, but also find therapy where you can.





