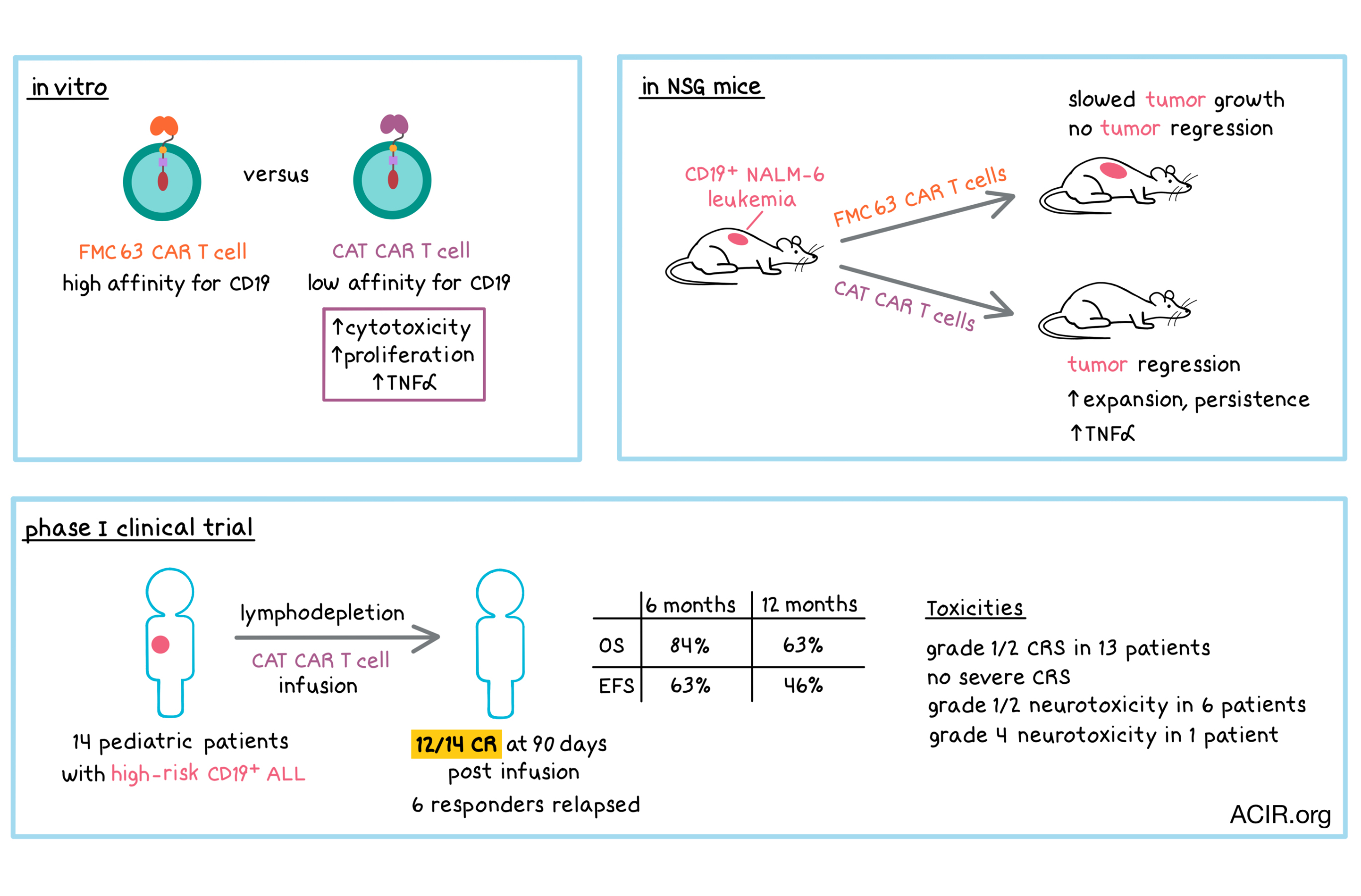
Aiming to determine how the affinity of a CAR for its target impacts efficacy and toxicity associated with CAR T cell treatment, Ghorashian et al. developed a low-affinity single-chain variable fragment (scFv) targeting CD19, compared it directly to the high-affinity scFv in a currently approved CAR product in vitro and in mice, and conducted a clinical trial in pediatric patients with acute lymphoblastic leukemia (ALL). The results were recently published in Nature Medicine.
The researchers began by generating CAT, a CD19-targeting scFv with >40-fold lower affinity for CD19 than FMC63-derived scFvs, which has been extensively tested clinically. Ghorashian et al. observed that CAT and FMC63 bound to the same or overlapping epitopes on CD19. Both CAT and FMC63 CAR constructs followed the same format: CD8-derived stalk and transmembrane domains, 4-1BB costimulatory domain, CD3ζ chain signaling domain, and an mCherry fluorescent protein used as a marker of transduction.
In vitro, CAT CAR T cells were more cytotoxic than FMC63 CAR T cells against a cell line with a high level of CD19 expression; both types of CAR T cells were equivalently cytotoxic against cells expressing CD19 at a low density. In coculture with CD19+ cells, CAT CAR T cells were more proliferative and secreted more TNFα than FMC63 CAR T cells. Production of other cytokines was similar between the two types of CAR T cells.
In immunodeficient NSG mice bearing NALM-6 leukemia, CAT CAR T cells led to tumor regression while FMC63 CAR T cells slowed tumor growth, but did not lead to tumor regression. Concordant with these observations, a higher number of CAT CAR T cells than FMC63 CAR T cells were found in the bone marrow (BM) and blood at 16 days after infusion. Increased levels of CD127 (IL7-Rα, which promotes proliferation) and Bcl-2 (which prevents apoptosis) may have contributed to enhanced expansion and persistence of the CAT CAR T cells. Additionally, confirming in vitro observations, CAT CAR T cells expressed higher levels of TNFα in vivo. Overall, preclinical results demonstrated that low-affinity CAR T cells exhibited enhanced antitumor response and expansion compared with high-affinity CAR T cells. Based on these results, Ghorashian et al. initiated a clinical trial.
In an open-label, phase I clinical trial, 17 patients under the age of 25 with advanced, high-risk CD19+ ALL were enrolled. Fourteen patients received lymphodepletion followed by an infusion of CAT CAR T cells (product could not be generated for the remaining 3 patients).
Thirteen out of 14 patients developed cytokine release syndrome (CRS), which was generally mild in nature (9 patients with grade 1, 4 patients with grade 2). No severe CRS was observed. Most patients did not have elevated levels of IFNγ, IL-6, or IL-10 cytokines, and the minority of patients with increased cytokine levels saw only modest increases. Six patients had grade 1/2 neurotoxicity, which was generally mild. One patient experienced grade 4 encephalopathy. Cytopenias were common (particularly for neutrophils), which may have been related to prior treatment and lymphodepletion. One patient developed prolonged neutropenia, multiple infections, and grade 4 encephalopathy, and died from sepsis while in remission. Thirteen out of 14 patients developed prolonged B cell aplasia, which is consistent with persistence of CAR T cells.
At 30 days post infusion, 10 patients were in complete remission (CR). At 90 days, 12 of 14 patients had achieved CR, one patient was alive with CD19+ disease, and one patient had died due to progression of CD19+ disease. Six patients who achieved CR ultimately relapsed: 5 with CD19- disease and 1 with CD19+ disease. In cases of CD19+ relapse/non-responding disease, anti-CAR-specific cytotoxic responses were detected, suggesting endogenous T cell-mediated rejection of CAR T cells. At a median follow-up of 14 months, 5 out of 14 (36%) patients remained in CR. Overall survival was 84% at 6 months and 63% at 12 months. Event-free survival was 63% and 46% at 6 and 12 months, respectively. These results, in a small number of patients, were comparable with the efficacy results observed in larger studies of approved CAR products.
In addition to safety and efficacy, Ghorashian et al. also examined what happened to CAR T cells in the body over time. CAR T cells exhibited mostly central memory or naive phenotypes and had a low level of dual expression of PD-1 and TIM3. CAR T cells underwent robust expansion in the periphery in 12 out of 14 patients, with a median time to peak expansion of 14 days. At peak expansion, a median of 41% of circulating T cells were CAR T cells, as determined by flow cytometry. CAR T cells contracted over time, but remained detectable in 11 out of 14 patients at last follow-up (up to 24 months for some patients). The median duration of persistence of CAR T cells at data cutoff was 215 days. Peak expansion, cumulative exposure, persistence, and half-life were all higher than published data for an approved CAR product, consistent with the in vitro and mouse data.
Overall, Ghorashian et al. demonstrated that T cells transduced with a low-affinity CD19 CAR exhibited enhanced expansion and persistence in vitro, in mice, and in patients, and enabled a strong antileukemic response with low toxicity in a clinical trial of pediatric patients with high-risk ALL. The authors caution that the safety profile in this clinical trial needs to be interpreted carefully, as the majority (10 of 14) patients had a low leukemic burden, which is associated with a lower risk of severe CRS.
by Anna Scherer
Meet the researcher
This week, first author Sara Ghorashian answers our 3 questions.

What prompted you to tackle this research question?
CAR T cell therapy for ALL was still in early development when we started the work on this study in 2014. It was clear that it could be effective but the toxicities were just being highlighted. We reasoned that some of the side effects of CAR T cell therapy may be related to characteristics of their activation and wondered if lower affinity CAR T cells may actually have advantages in terms of persistence and toxicity.
What was the most surprising finding of this study for you?
That our assumptions were generally correct: we saw equivalent efficacy, improved CAR T cell expansion and persistence, and lower toxicity compared to Kymriah, Novartis’s licensed CAR T cell product when treating patients with advanced ALL. However, in terms of toxicity, patients who were treated had a lower bone marrow disease burden than those treated with Kymriah on the ELIANA study, and this may also have played a role in the lower levels of toxicity seen.
What was the coolest thing you’ve learned (about) recently outside of work?
Given the current affairs I would turn the question around: what is the least cool thing you’ve read about? Anything to do with Brexit!




