Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer death, and while surgery can be curative, the vast majority of patients relapse and succumb to their disease, even when given additional chemotherapy. While PDAC is generally resistant to immune checkpoint inhibitors, recent studies have shown that they do often harbor immunogenic neoantigens that could serve as targets for vaccination. Based on this, Rojas and Sethna et al. utilized surgically resected PDAC samples to manufacture and deliver personalized neoantigen-targeting vaccines based on a uridine mRNA and lipoplex nanoparticle platform. The results of this phase I study were recently published in Nature.
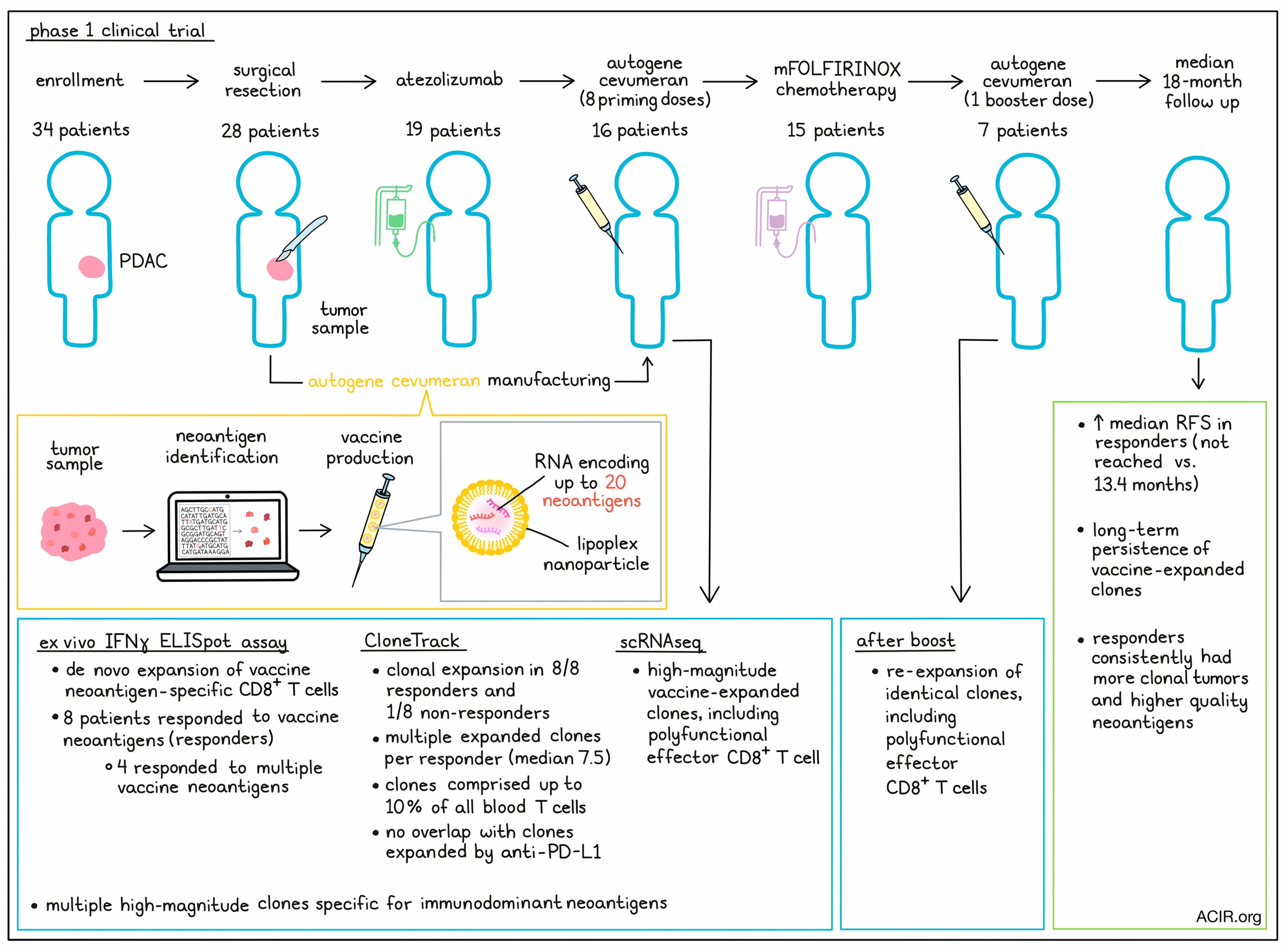
For this trial, Rojas and Sethna et al. enrolled 34 patients, 28 of whom underwent surgery. 6 weeks after surgery, 19 patients were treated with a single dose of atezolizumab (anti-PD-L1), and 3 weeks later, 16 patients received up to 8 priming doses of their personalized autogene cevumeran vaccine, which contained RNA encoding up to 20 neoantigens per patient. 15 of the patients who received their vaccines were subsequently treated with mFOLFIRINOX, a modified version of a four-drug chemotherapy regimen, consisting of folinic acid, fluorouracil, irinotecan, and oxaliplatin. Several patients also received a late booster dose of vaccine. All patients were treated and monitored at Memorial Sloan Kettering Cancer Center.
Looking at safety, none of the 19 atezolizumab-treated patients had grade 3 or higher adverse events (AEs), and only 1 of 16 patients had a grade 3 AE following autogene cevumeran. All patients had grade 1 and 2 AEs, but overall, treatments were tolerable.
Next addressing feasibility, the researchers were able to manufacture autogene cevumeran and administer it within 3 days of their benchmarked times, a median of 9.4 weeks after surgery. Only one patient had insufficient antigens for vaccine manufacturing. 3 out of 16 patients did not receive all 9 doses of their vaccine due to disease progression, death, or mFOLFIRINOX toxicity.
To measure vaccine-induced T cell responses, Rojas and Sethna et al. used an ex vivo IFNγ ELISpot assay and found that autogene cevumeran induced de novo high-magnitude neoantigen-specific T cells in 8/16 patients, deemed responders. Ex vivo responses were detected against 25 of 230 neoantigens administered across all patients, with 4 patients showing evidence of responses to multiple neoantigens.
To further evaluate T cell responses, the researchers developed CloneTrack – a tool that identifies clonal expansion and abundance by comparing T cell receptor (TCR) Vβ sequencing of peripheral blood before and after treatment. Using this system for evaluable patient samples, vaccine-induced clonal expansion was identified in 8 out of 8 ELISpot responders and 1 out of 8 non-responders. Multiple expanded clones were identified in each responder (median 7.5), that comprised up to 10% of total blood T cells. When CloneTrack was applied to samples collected before and after atezolizumab treatment, there was no overlap between atezolizumab-expanded clones and vaccine-expanded clones.
Next, the researchers used in vitro assays to identify T cell clones specific for ELISpot-identified immunodominant neoantigens. Looking at the clonal overlap with clones expanded by autogene cevumeran in vivo, 21 out of 41 vaccine-expanded high-magnitude clones across 3 out of 4 evaluated patients were specific for ELISpot-identified immunodominant neoantigens. In the fourth patient, the immunodominant neoantigen-specific clones were still present, but fell into a lower-magnitude vaccine-expanded clonal pool.
Single-cell RNAseq revealed that high-magnitude vaccine-expanded clones were predominantly CD8+ T cells, which expressed markers of effector function, including perforin 1, granzyme B, and IFNγ. In samples taken after vaccination, CD8+ T cells, but not CD4+ T cells, were found to be polyfunctional, and produced IFNγ and granzyme B upon ex vivo rechallenge with long neopeptides and MHC-I-restricted minimal epitopes.
When patients were treated with a vaccine booster at 46 weeks post-surgery, clones previously expanded by priming doses were re-expanded in all responders who received boosters. This expanded T cell population included polyfunctional, neoantigen-specific effector CD8+ T cells. Despite mFOLFIRINOX treatment, T cells maintained the capacity to produce IFNγ upon stimulation. Further, vaccine-expanded clones showed long-term persistence, making up as much as 2.5% of total blood T cells for up to 2 years post-surgery.
At a median follow-up of 18 months, responders to autogene cevumeran had longer median recurrence-free survival (RFS) than non-responders (not reached vs. 13.4 months). A time-to-response bias was excluded by correlating RFS to response in patients who were recurrence-free throughout all 8 vaccine priming doses. Responders showed persistently lower serum CA19-9 levels – a clinical biomarker of PDAC.
To identify whether vaccine responses were simply better in patients with already better prognosis, the researchers looked at various clinical factors, including response to atezolizumab, lymph node positivity, margin positivity, primary tumor size, the number of chemotherapy doses, and the density of intratumoral CD8+ T cells. None of these variables were found to correlate with vaccine response. Responders and non-responders also had comparable immunological fitness, with similar frequencies of innate and adaptive immune cells in the periphery, and similar responses to RNA vaccines for SARS-CoV-2.
In search of correlates of response, Rojas and Sethna et al. examined the relationship between vaccine-induced responses and tumor clonality and neoantigen quality. Quality was evaluated using algorithms that incorporate the relationships of the neoepitope to known immunogenic epitopes and to self-epitopes. Responders consistently had more clonal tumors than non-responders, and their tumors contained neoantigens with high-quality features that correlated with vaccine neoantigens capable of eliciting T cell IFNγ responses.
In one responding patient, the researchers observed an increase in serum CA19-9, which coincided with the appearance of a 7mm liver lesion. Suspecting metastasis, the researchers biopsied the lesion, and found that it did not contain malignant cells, but rather a dense aggregate of lymphoid cells that contained all 15 vaccine-expanded CD8+ T cell clones for that patient, which showed evidence of lytic and effector potential. They also identified a few rare cells harboring the TP53 R175H mutation, which was identical to the R175H driver mutation in the patient’s primary tumor. This lesion disappeared on later imaging, suggestive of immune-mediated elimination of a potential micrometastasis.
Overall, this trial successfully met its endpoints of safety, feasibility, induction of vaccine-induced neoantigen-specific T cells, and 18-month recurrence-free survival for personalized vaccination with adjuvant autogene cevumeran. In combination with anti-PD-L1 and mFOLFIRINOX, this treatment induced strong tumor-specific responses and delayed tumor recurrence in patients with PDAC, providing a glimmer of hope to improve outcomes for this deadly disease.
Write-up and image by Lauren Hitchings




