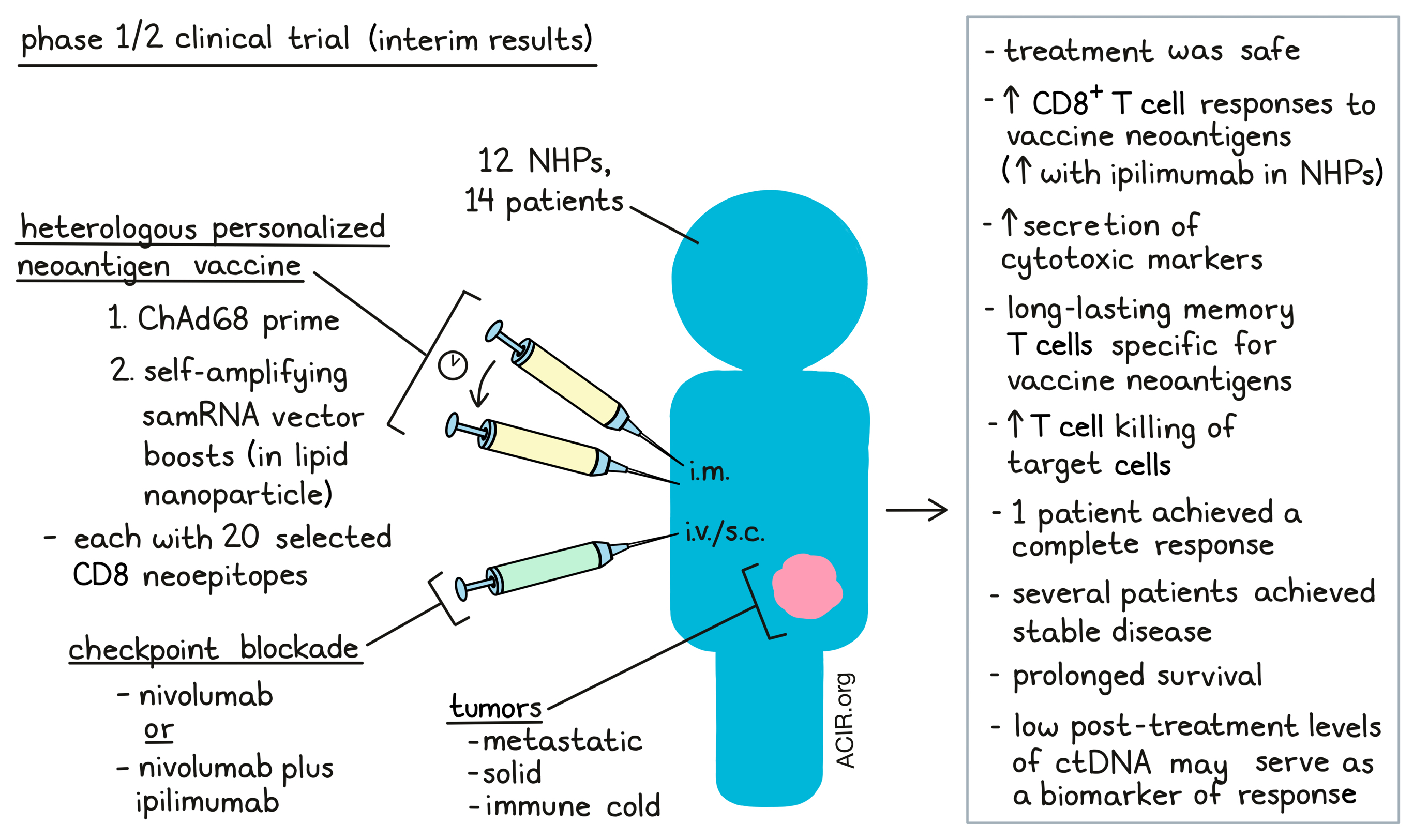
Given that checkpoint inhibition therapies have limited efficacy in tumors poorly infiltrated with T cells (immune cold tumors), combining these therapies with those attempting to increase antitumor T cell responses, such as vaccines, is warranted. Palmer et al. performed a phase 1/2 clinical trial assessing the safety and efficacy of an individualized neoantigen vaccine strategy combined with checkpoint inhibition in patients with various metastatic solid tumors. The interim results from this trial were recently published in Nature Medicine.
The heterologous prime-boost vaccine consisted of a chimpanzee adenovirus (ChAd68) and a fully synthetic Venezuelan equine encephalitis virus-based self-amplifying mRNA (samRNA) in a lipid nanoparticle. The researchers started out by assessing the potency and durability of immune responses obtained by this vaccine strategy in non-human primates (NHP). To do so, a model antigen cassette encoding six NHP-specific MAMU-A*01-restricted simian immunodeficiency virus (SIV) antigens was inserted into the vaccine platforms, and MAMU-A*01-positive rhesus macaques were injected intramuscularly (i.m.) with the ChAd68 prime, as well as samRNA boosts at 4, 12, and 20 weeks, with or without subcutaneous (s.c.) ipilimumab (anti-CTLA-4).
The ChAd68 prime induced immune responses against all six antigens, and these primed T cell responses were boosted by the samRNA, remaining detectable at 32 weeks. The addition of ipilimumab elevated T cell responses about 2-3 fold, which could be further elevated by readministration of ChAd68 at week 32. Antigen-specific effector memory T cells were generated and maintained over treatment. Six animals were studied for two years after prime vaccination alone using tetramer analysis of peripheral blood mononuclear cells (PBMCs). Antigen-specific central memory T cells were observed that had reactivity against four of the six antigens in these samples. Readministration of samRNA at this timepoint boosted antigen-specific T cell levels about 10-fold. Finally, CD8+ T cells from all six animals expressed cytotoxic activity against target cells.
The vaccine strategy was then assessed in a still ongoing phase 1/2 trial in patients with metastatic microsatellite-stable colorectal cancer (MSS-CRC, N=7)), non-small cell lung cancer (NSCLC, N=1), and gastroesophageal adenocarcinoma (GEA, N=6). Patients received i.m. injections of a single priming dose of ChAd68, followed by multiple i.m. boosts with escalating doses of the samRNA. The 20 highest-ranking mutations in the patient’s tumor tissue were selected for inclusion in a codon-optimized vaccine expression cassette; each mutation was encoded as a 25mer encompassing the mutation. Fourteen patients received the vaccination, as well as monthly intravenous (i.v.) nivolumab (anti-PD-1) beginning at the time of the first vaccine dose. Furthermore, patients treated at higher dose levels also received s.c. ipilimumab with each vaccination. There were no dose-limiting toxicities with this treatment. Treatment-related adverse events were consistent with vaccine-induced immune reactions and checkpoint inhibitor treatment.
The researchers assessed efficacy by analysis of neoantigen-specific T cells. For this, minimal epitope (8-11mer) peptide pools were designed for each patient with the 40 highest-ranked predicted neoepitopes (CD8 pool) and an additional pool with overlapping 15mers spanning each vaccine cassette (15mer pool). PBMC samples from 13 patients were stimulated with the peptide pools. This resulted in detectable IFNγ responses in all patients after the prime-boost, which was further enhanced in 67% of patients by a ChAd68 boost, which also elevated T cell levels. Long-lived T cell responses were maintained for over a year in three patients with available samples. Due to COVID-19 measures at the time of the study, some patients received the samRNA dosing at prolonged intervals. This, combined with the dose escalation study, revealed that lower doses and longer intervals between boosts may be beneficial for T cell responses, which is now being assessed in the ongoing phase 2 of this study.
To assess the breadth of the immune response to vaccination, four minipools covering the 20 patient-specific vaccine neoantigens were used to deconvolute T cell responses. All patients had detectable T cell responses to multiple mutations after treatment. Assessment against all 40 minimal epitopes included in the CD8 pool for three patients showed responses to over 50% of vaccine mutations in multiple HLA class I alleles for all three patients. Therefore, partial deconvolution via minipools (mixtures of 5 peptides) may underestimate the extent of the responses. Peptide HLA (pHLA) tetramer-positive CD8+ T cells were more commonly effector memory and less commonly naive when compared to total CD8+ T cells.
Secretion of various cytotoxic markers was detected in eight patients, and readministration of ChAd68 increased granzyme B secretion in response to CD8 pool stimulation in 4/6 patients. Stimulation of PBMCs with the CD8 pool induced expression of one or more of IFNγ, CD107α, TNFα, and/or IL-2 on CD8+ T cells. Additionally, co-culture of expanded neoantigen-specific T cells with single HLA allele-expressing target cells pulsed with cognate neoantigen peptides revealed T cell killing of target cells.
For two patients, baseline and on-treatment PBMCs were available for paired T cell receptor (TCR)seq and single-cell RNAseq. Increases in neoantigen-specific CD8+ T cells were detected after treatment, resulting in an enrichment of CD8+ T cells in treatment samples. Distinct TCR clonotypes were detected, and these T cells had increased expression of IFNG, GZMB, and/or TNFA transcripts. Putative neoantigen-specific T cells were detected in both the tumor and blood, suggesting vaccine-induced neoantigen-specific T cells infiltrated the tumor.
Finally, Palmer et al. assessed the clinical efficacy of this vaccination strategy. Patients with MSS-CRC and GEA had tumors with a low mutational burden, low PD-L1 expression, and limited infiltration of immune cells prior to treatment (immune cold tumors). One patient with GEA had a complete response and several patients in the study experienced stable disease (SD). The patients with MSS-CRC were further analyzed for overall survival (OS) and circulating tumor (ct)DNA. The 12-month OS rate was 42.9%, and patients with longer OS had lower post-treatment ctDNA levels. Patients with SD lasting <6 months had increased ctDNA concentrations, while 75% of patients with longer SD had lower levels of ctDNA, including two patients with complete ctDNA clearance, suggesting low ctDNA levels may be a biomarker for treatment efficacy.
In conclusion, these interim data suggest that this vaccine regimen is tolerable and may increase T cell immune responses in immune cold tumors. Further efficacy data will be obtained in the ongoing clinical trials with the determined dosing for this regimen.
Write-up by Maartje Wouters, image by Lauren Hitchings.




