The simple hypothesis that NK cells discriminate “self” from “non-self” based on surface HLA expression understates the complexity of NK cell recognition, and now needs to be extended to include discrimination of “altered self”. As the recent study by Carrillo-Bustamante et al. suggests, changes in the peptide-ligand repertoire appear to play a role in NK cell regulation as well.
NK cell recognition of target cells is tightly controlled by the interactions between the inhibitory and activating killer-cell immunoglobulin-like receptors (KIRs) and their corresponding ligands - the most common ones being HLA-A3/11, HLA-B Bw4, HLA-C C1, and HLA-C C2. Malmberg et al. describe that the KIR gene family has up to 17 members, which vary in number between individuals and exhibit allele-specific polymorphisms. As explained in a review of KIR evolution by Hilton and Parham, the extensive genetic diversity and polymorphism of both the KIR and HLA class I genes result in a diverse NK cell response among individuals, differentially impacting sensitivity to infection, autoimmunity, and reproduction.
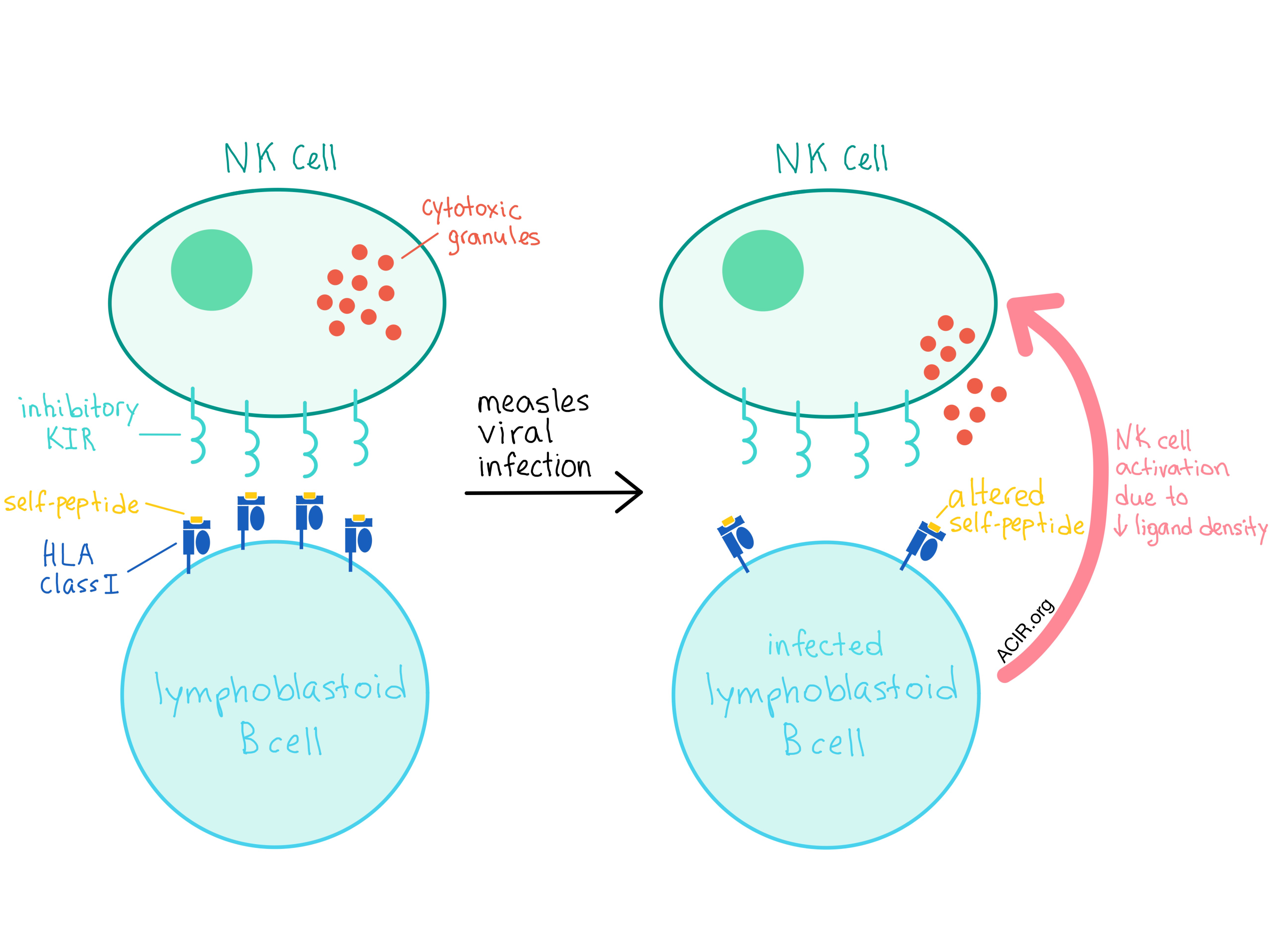
In addition to the allele-generated diversity, there is now increasing evidence that NK cells utilize HLA-bound peptide selectivity (possibly at positions P7 and P8) as another level of control for inhibition or activation. In an attempt to quantify the specificity required at the ligand-peptide level for NK activation and how this relates to inhibitory KIR (iKIR) genetic diversity, Carrillo-Bustamante et al. evaluated the changes in the repertoire of the peptide-HLA class I complexes that occur during measles viral infections in a set of B-cell lymphoblastoid cell lines (BLCLs).
The researchers found that the number of unique peptides decreased in all four BLCLs after infection with a measles virus, although the total number of peptides increased in two of the cell lines. Surprisingly, the density of most ligand sequence motifs at P7 and P8 decreased in all cell lines. As a decreasing ligand density leads to enhanced NK cell activation, the observed large decrease in ligand concentration is likely to trigger a strong NK cell response. Interestingly, the majority of peptides detected after viral infection were derived from self-proteins, while only a few were of viral origin, implying that changes in self-peptide presentation may be sufficient to detect a viral infection.
The analysis performed by Carrillo-Bustamante et al. implies that iKIRs could only detect the changes in the peptide-HLA class I repertoire if the iKIRs were sufficiently specific (i.e., able to distinguish between different amino acids in the contact residues of the peptide), and the simulation model utilized by the researchers led to the conclusion that one single iKIR per individual host would be insufficient to detect repertoire changes, which may explain the evolution of the KIR multigene family. The validity of these conclusions depends crucially on the actual specificity of iKIRs, which remains unknown at this point.
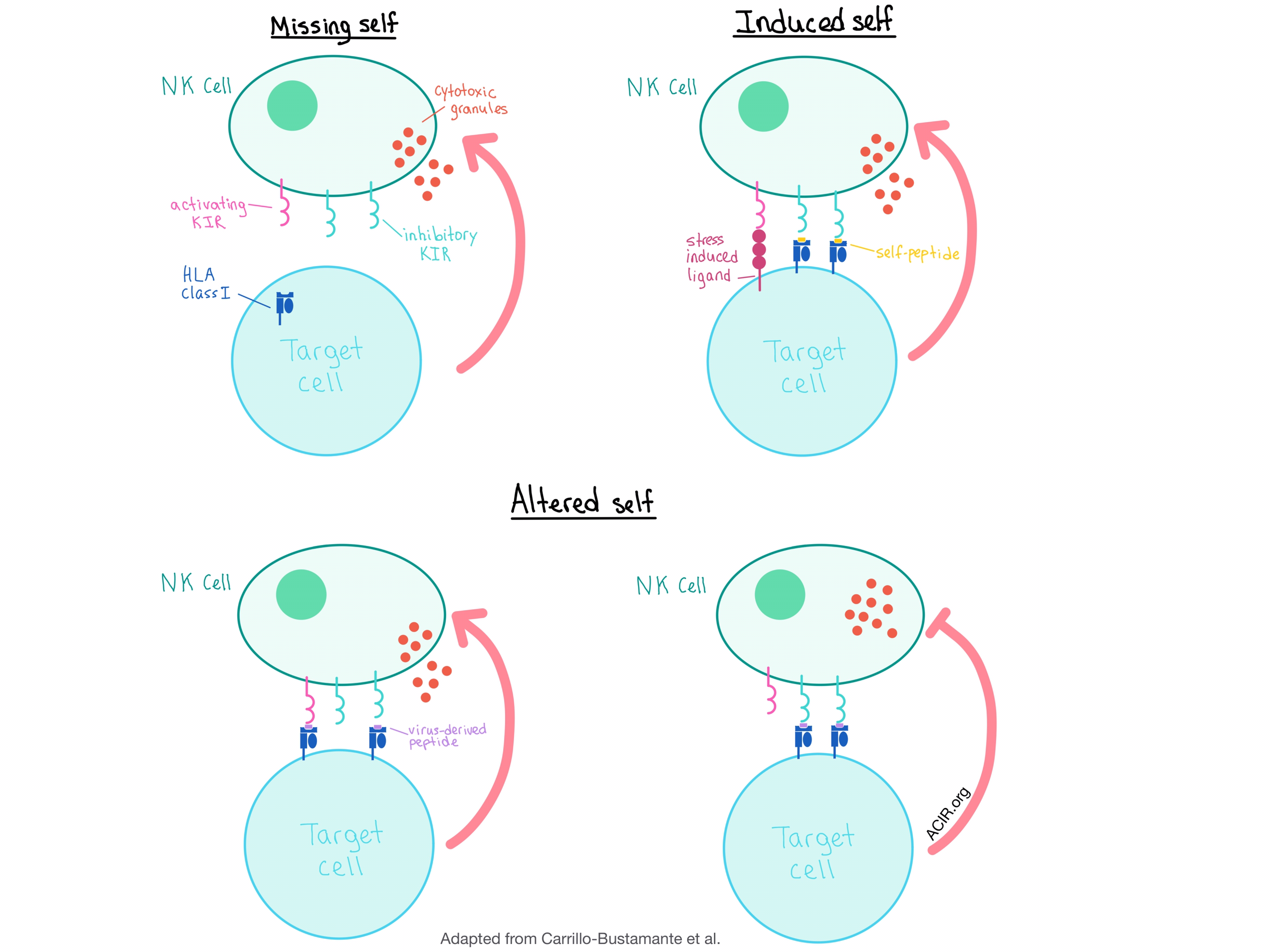
The results of this study, together with previous research, suggest that the current model of NK cell activation, which includes the detection of “missing self” (no HLA expression) and “induced self” (activating ligands), should be augmented to include the “altered self” scenario, in which virus-induced peptide-HLA class I repertoire changes can activate NK cells by either promoting binding to the activating KIRs or disrupting binding to the iKIRs, even in the absence of HLA class I downregulation. Alternatively, the target cell could inhibit NK cell activation by presenting peptide:HLA complexes that bind strongly to the iKIRs, allowing the virus (or a tumor) to escape detection.
Finally, as mentioned in a review by Malmberg et al., NK cells have the potential to play a role in allogeneic adoptive immunotherapy, particularly in converting patients with acquired resistance to checkpoint inhibition therapy via HLA loss into clinical responders. Terminally differentiated memory NK cells (a.k.a. adaptive NK cells) have the advantage of homogeneous expression of a single HLA-specific self-KIR, rendering these cytolytic cells completely safe for normal tissue not expressing the target HLA and making them attractive candidates for cancer treatment.
Despite the challenges that remain to fully understand the mechanism behind NK cell specificity and functionality, the work by Carrillo-Bustamante et al. brings scientists one step closer to utilizing this highly active immune cell population to enhance cancer immunotherapy.
by Anna Scherer




