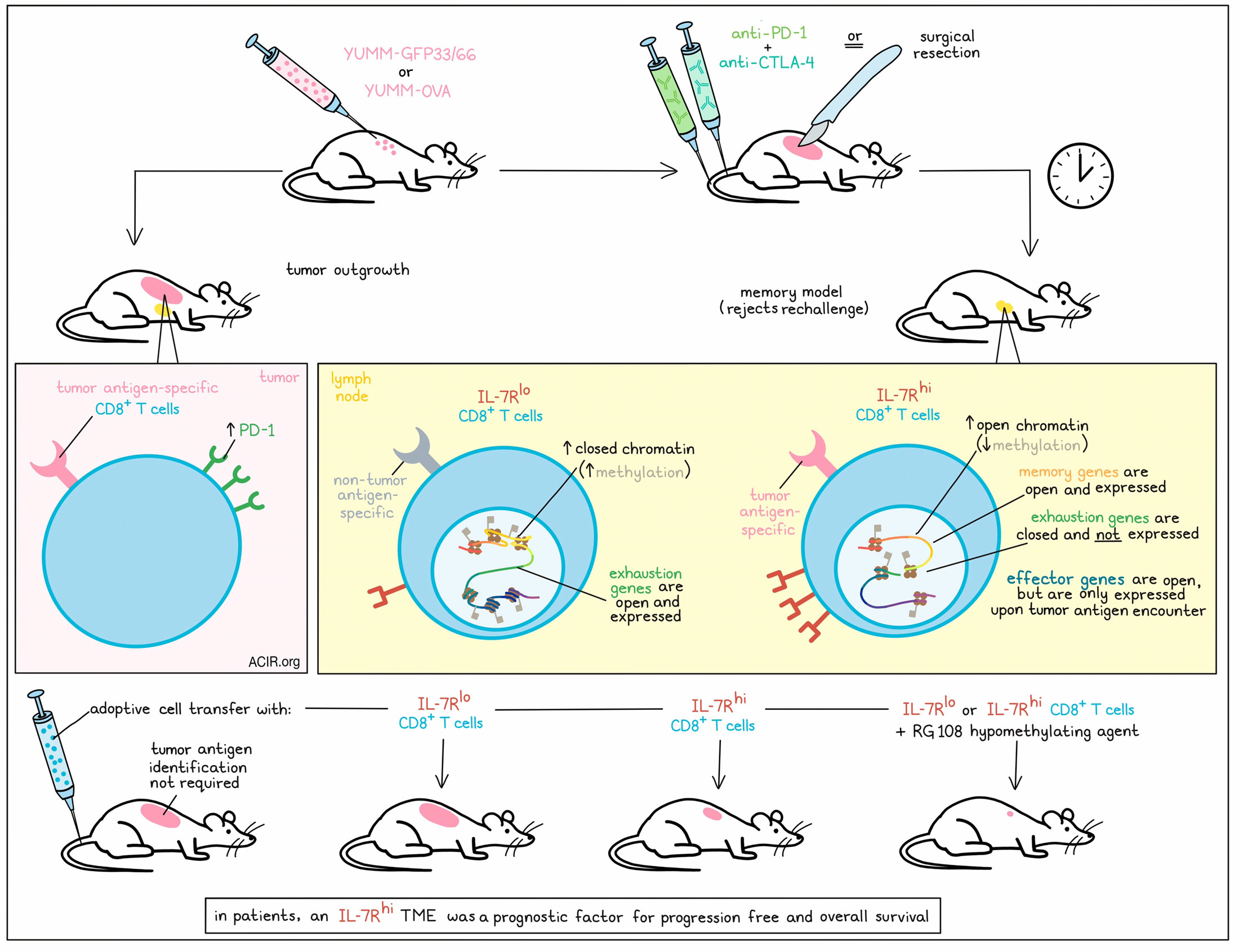
Melanomas are often immunogenic and responsive to surgical resection and checkpoint blockade therapies; however, tumor recurrence is still a common problem. To better understand and ultimately prevent tumor recurrence, Micevic and Daniels et al. recently identified a population of L-7Rhi CD8+ T cells that is mostly tumor-specific, is maintained in lymph nodes long-term, and is capable of mediating strong cytotoxic memory responses against recurring melanomas. This cell state was found to be epigenetically regulated, and could be enhanced through pharmacological epigenetic modulation. The results were recently published in PNAS.
To study immune memory and melanoma recurrence, Micevic and Daniels et al. used the YUMM1.77 melanoma model expressing either LCMV gp33-41 and gp66-77 antigens and a fluorescent label (YUMM-GFP33/66) or OVA (YUMM-OVA). These models respond to surgical resection or combination checkpoint blockade (anti-PD-1 plus anti-CTLA-4), establishing models of immune memory that reject rechallenge in a tumor-specific and T cell-dependent manner. Using this model, the researchers detected dominant antigen-specific T cells that expanded in tumor-draining lymph nodes (tdLNs) and spleens after tumor challenge, and expanded proportionally in tdLNs for 6 weeks after tumor resection. Blocking lymphocyte egress from LNs abrogated memory responses. Bulk RNAseq of CD8+ T cells from lymphoid organs revealed a memory-like transcriptional profile, including high expression of IL7r.
Given that IL-7R marks several cell states and is critical for T cell development, survival, and memory differentiation, the researchers further investigated its expression in tdLN populations in memory models. While tumor-specific CD8+ TILs cells from mice with progressing tumors showed increased expression of PD-1, over 90% of tumor-specific T cells from the lymph nodes of memory model mice were marked by high expression of IL-7R. By contrast, only 3% of non-tumor-specific CD8+ T cells expressed IL-7R in this model, suggesting that IL-7R could be used as a marker for tumor-specific T cells in the memory setting. Blocking IL-7R during initial tumor challenge, but not during rechallenge, reduced functional memory responses and survival, suggesting that IL-7R is functionally important in the establishment of immune memory.
Next, the researchers used scRNA and clustering analysis to further characterize a cluster of CD8+ T cells with high IL-7R expression. This cluster was enriched for central-memory-like and cytotoxic-like T cell phenotypes, and expressed Ccr7, Sell, Cd27, Cd28, Tcf7, Eomes, and Bcl-2. It also had the highest overall cytotoxicity score (along with the cluster of NK cells) and expressed several intrinsic cytotoxic mediators, including Nkg7. Interestingly, genes canonically associated with a CD8+ T cell effector signature, such as Gzmb, Prf1, and Ifng, were not highly expressed in the IL-7Rhi CD8+ T cell cluster.
Because of the strong overlap between high IL-7R expression and tumor antigen specificity, the researchers evaluated IL-7Rhi cells as candidates for adoptive cell transfer (ACT). While transfer of IL-7Rlo cells was comparable to negative controls, the transfer of IL-7Rhi cells (isolated from tdLNs of mice with immune memory) reduced tumor growth and prolonged survival by 50%, inducing cures in a subset of mice. Importantly, this method did not require the identification of any specific tumor antigens.
Epigenetically, the researchers observed significantly lower DNA methylation on the Tcf7 promoter in IL-7Rhi compared to IL-7Rlo cells, in line with methylation repressing gene expression. Further, chromatin was more open near the transcriptional start sites of Il7r, Tcf7, and other memory-associated loci, and more closed near genes associated with exhaustion. Interestingly chromatin also remained open near canonical effector loci associated with cytotoxicity and production of IFNγ, IL-2. TNFɑ, granzyme A, granzyme B, and perforin. While cytotoxic molecules were not actively expressed in the memory setting, they were found to be upregulated in this same population immediately after rechallenge, suggesting that IL-7Rhi cells are epigenetically poised to respond to tumor rechallenge.
Treating TILs ex vivo with the hypomethylating agent RG108 increased their expression of Tcf7 and Il7r. Investigating whether this could enhance ACT, the researchers treated IL-7Rhi or IL-7Rlo CD8+ T cells populations with RG108 ex vivo and then transferred them into naive mice. Mice were then challenged with YUMMER1.7 (a high-antigen-load variant of YUMM1.7, but without a dominant antigen [CMV or OVA]). Compared to untreated IL-7Rhi cells, transfer of RG108-treated IL-7hi cells conferred enhanced protective immunity, extending median survival and inducing tumor clearance in 75% of mice. Further, RG108-treated IL-7lo cells also conferred enhanced antitumor immunity, abrogating the difference between IL-7Rhi and IL-7Rlo populations that was previously observed.
Investigating the translational relevance of these findings, Micevic and Daniels et al. analyzed IL-7R expression in the TME in a large cohort of patients with melanoma. Like in mice, human IL-7Rhi CD8+ T cells exhibited hypomethylation of the Tcf7 promoter, corresponding with higher TCF7 expression compared to the IL-7Rlo group. Human IL-7Rhi CD8+ T cells also expressed the same memory-like methylation signature that was observed in mouse models.
To determine whether IL-7R expression was associated with durable responses and survival, the researchers stratified a large cohort of patients with advanced melanoma into IL-7Rhi or IL-7Rlo TME groups. The IL-7Rhi cohort was enriched for expression of genes associated with T cell signaling and immune responses, and showed prolonged progression-free (755 vs. 523 days) and overall (2,421 vs. 875 days) survival. This correlation could not be attributed to any other prognostic factors, and IL-7R expression was determined to be an independent prognostic factor for overall survival. Interestingly, TCF7 did not correlate with survival, suggesting that IL-7R expression was not simply a reflection of increased stemness. Similar results were observed in a large cohort of patients with lung cancer, and in a 60-patient cohort of patients with melanoma who were treated with checkpoint inhibitors.
To understand how IL-7R impacts response to anti-CTLA-4 and anti-PD-1 therapy, tumor-bearing mice were treated with dual checkpoint blockade. While blocking IL-7R concurrently with immunotherapy did not affect the remission induced by anti-PD-1 and anti-CTLA-4, it did lead to enhanced tumor recurrence, suggesting a critical role for IL-7R signaling in maintaining durable antitumor immunity.
Overall, Micevic and Daniels et al. showed that high IL-7R expression is epigenetically regulated and plays an important role limiting tumor recurrence. IL-7Rhi CD8+ T cell populations are maintained in lymph nodes long-term, where they remain poised and ready to respond to any re-emerging tumor cells. Further, IL-7Rhi CD8+ T cells could have broad clinical applications, as they are largely tumor-specific, can be enhanced with hypomethylating agents, and could allow for effective ACT without the identification of specific tumor antigens.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, co-first authors Andrew Daniels and Goran Micevic answered our questions.
What was the most surprising finding of this study for you?
AD: One of the most surprising findings to us was the astounding efficacy of pre-treating T cells with hypomethylating agents at improving their antitumor functionality. Not only were hypomethylating agents able to bolster the potency of T cells that expressed functional markers prior to transfer to naive mice, but they were able to generate almost identical efficacy in the much larger population of T cells that lacked expression of these markers altogether.
GM: The ability of IL-7R blockade to abrogate the robust functional memory phenotype was also striking.
What is the outlook?
AD: These findings present an exciting area for clinical innovation. In recent years, we have gained a better understanding of some of the epigenetic mechanisms that contribute to the robust T cell reprogramming that happens in the setting of tumors. We saw adoptive cell therapies as one of the key treatment modalities that could benefit from leveraging some of these innovations.
GM: Further studies will be required to determine the critical epigenetic drivers and optimal ways to target them. Important questions regarding the “anatomy” of a successful antitumor memory response remain, including the timeline, signals, cell–cell interactions, and compartments where they occur.
What was the coolest thing you’ve learned (about) recently outside of work?
AD: It’s been all about foraging mushrooms for me! Many of the tastiest varietals you can harvest can’t be cultivated, due to the intricate relationships they form with trees in order to get their nutrients. This makes foraging a really cool opportunity!
GM: I recently learned that marine diatoms, single-cell algae, produce a significant portion of the oxygen we use! Like T cells, they are small, but mighty. This epithet also applies to my 3-year-old and is a reminder to cherish the amazing “little things” in life.




