Regulatory T cells (Tregs) are the best known immunosuppressive CD4+ T cell subset, but Tr1 T cells, which are CD4+Foxp3- and marked by high expression of IL-10, are also known to maintain tolerance in autoimmunity and chronic infections. In recent work, Sultan et al. identified a population of Tr1 cells in tumors that inhibited antitumor immunity under certain vaccine conditions, and in resistance to anti-PD-1. Their work identifying this population of Tr1 cells, parsing out their mechanisms, and exploring strategies to circumvent their inhibitory functions was recently published in Nature.
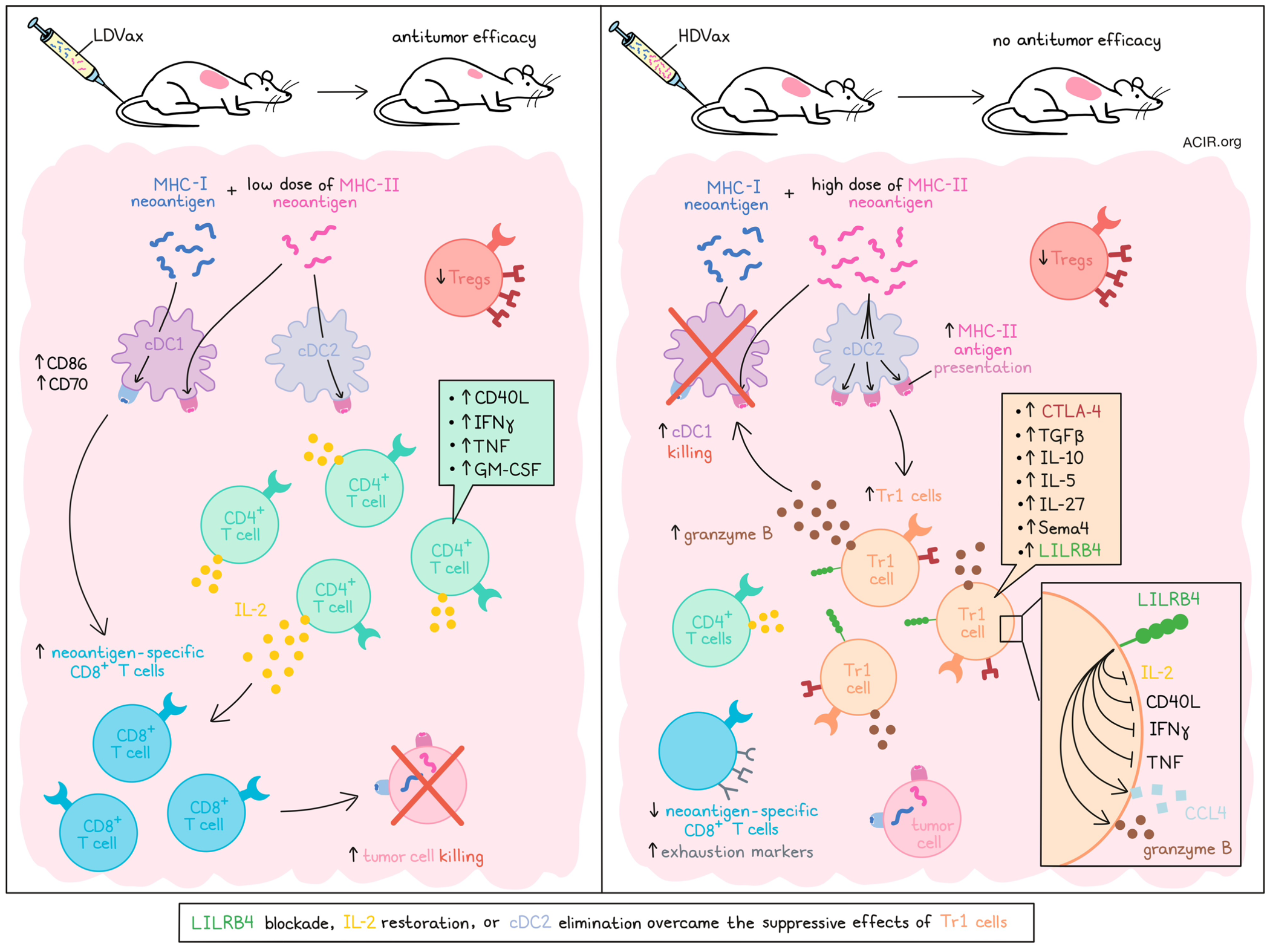
Sultan et al. utilized various MCA sarcoma lines (T3, F244, and 1956) for which the MHC-I and MHC-II unique major neoantigens have been identified. Testing various synthetic long peptide neoantigen vaccine formulations, the team found that in vaccines targeting a fixed dose of relevant MHC-I neoantigens, those with a low dose of relevant MHC-II neoantigens (LDVax) induced tumor clearance, while those with a high dose of relevant MHC-II neoantigens (HDVax) did not. Further, while LDVax had no impact when added to anti-PD-1, anti-4-1BB, or anti-CTLA-4 therapies (all of which induced antitumor effects as monotherapies), the addition of HDVax abrogated the antitumor effects of anti-PD-1 or anti-4-1BB, but not anti-CTLA-4. These inhibitory effects were antigen-specific, as HDVax only impacted the antitumor efficacy of checkpoint blockade in tumor lines that were matched with their relevant vaccine formulations.
To better understand the inhibitory the effects of HDVax, the researchers developed an adoptive cell transfer (ACT) model in which T cells induced by T3-targeted LDVax in wild-type mice were transferred into T3-tumor-bearing Rag2-/- mice. While cells from the LDVax-treated mice induced tumor rejection, the addition of Treg-depleted (using anti-CD25) MHC-II neoantigen-specific HDVax-induced cells abrogated this effect, indicating the presence of non-Treg inhibitory cells. While both HDVax and LDVax reduced Tregs and induced similar numbers of CD4+ TILs, phenotypic analyses revealed that neoantigen-specific CD4+ T cells induced by LDVax expressed IL-2, along with increased CD40L, IFNγ, TNF, and GM-CSF upon stimulation, while those induced by HDVax produced no measurable IL-2, had limited expression of CD40L, and expressed high levels of IL-10, TGFβ, IL-5, and IL-27. Further, DCs in LDVax-treated mice expressed more CD86 and CD70, while in HDVax-treated mice, cDC1s were reduced in number. In line with this, HDVax-treated mice also had fewer neoantigen-specific CD8+ T cells, which expressed higher levels of exhaustion markers (PD-1, CD39, Tim3, Lag3, and TOX) and produced less IFNγ and TNF than neoantigen-specific CD8+ T cells in LDVax-treated mice.
To identify the cellular subpopulations involved in these phenotypic differences, the researchers performed scRNAseq and UMAP clustering of neoantigen-specific CD4+ TILs from T3 tumors following different vaccinations. This revealed several differences in clustering, with one cluster (Cluster 3; Gzmb+/Ccl5+) that was greatly increased in density following HDVAx, but not LDVax. HDVax-induced CD4+ TILs expressed relatively high levels of Pdcd1, Tox, and Vsir transcripts, and lower levels of Lag3, Cd200, Havcr2, Tigit, Ifng, Tnf, Cd28, Cd40l, and transcription factors associated with TH1 cell polarization. Additionally, gene set enrichment analysis revealed defects in the IL-2 signature. These results were validated at the protein expression level, which also revealed higher CTLA-4 protein in HDVax-induced CD4+ T cells, which could explain why HDVax did not inhibit anti-CTLA-4, as this population would likely have been depleted.
While Sultan et al. could not initially identify specific proteins that would isolate cluster 3, which they suspected to be largely responsible for the differential phenotypes observed in HDVax-induced TILs, depleting cells with markers not expressed in cluster 3, and then selecting for CD39 allowed the team to isolate a partially purified cluster 3 population. This population showed stronger suppressive effects than Tregs in vivo, and was found to be marked by Lilrb4 and Sema4a expression, with Lilrb4 proving to be essential to the population’s inhibitory functions. Further, Foxp3-LILRB4+ T cells were also found to be increased in progressively growing, untreated T3 tumors, and appeared in anti-PD-1-treated tumors upon the onset of resistance. This population was found to be neither a precursor to, nor derivative of Tregs, and instead overlapped with the identities of Tr1 and cytotoxic CD4+ T cells, with LILRB4+ cluster 3 cells demonstrating Tr1 hallmarks, including high expression of IL-10 and low expression of IL-2, TNF, and GM-CSF.
In an effort to reduce the inhibitory effects of these newly identified tumor-infiltrating Tr1-like cells, the researchers found that while IL-10 blockade was ineffective, LILRB4 blockade or deletion reduced the inhibitory capacity of this cell population in ACT models of combined LDVax- and HDVax-induced T cells. LILRB4 blockade even supported the antitumor efficacy of HDVax alone in WT mice. Rather than depleting Tr1 cells, anti-LILRB4 (or genetic deletion of LILRB4) appeared to decrease Tr1 production of CCL4 and granzyme B, restore IL-2 expression, and increase expression of IFNγ, TNF, and CD40L. Anti-LILRB4 also increased neoantigen-specific CD8+ T cells and reduced their expression of exhaustion markers. Restoring IL-2 using a CD8–IL-2 mutein (to limit IL-2-associated toxicity) had a similar effect to anti-LILRB4 in limiting the suppressive impacts of Tr1 cells and enabling the antitumor efficacy of HDVax.
Looking at differences in APCs induced by LDVax versus HDVax, the researchers found that in LDVax-treated tumors, cDC1s and cDC2s presented the MHC-II neoepitope to similar extents, while in HDVax-treated tumors, cDC2s acted as the primary APCs for MHC-II neoepitopes. This was found to be related to Tr1-mediated killing of cDC1s in a Gzmb-dependent manner, and could be abrogated with anti-LILRB4. Anti-CTLA-4, which presumably depleted both Tr1 cells and Tregs, also restored cDC1s. Further, in mice that were unable to generate cDC2s, HDVax did not induce the development of the Tr1 subset, cDC1s were not reduced, and the antitumor effect resembled that of LDVax. Further, in mice unable to generate cDC2s, Tr1 cells did not accumulate in progressing tumors or in tumors becoming resistant to anti-PD-1. In the same model, anti-PD-1 maintained efficacy when started at later time points in established tumors.
Together, these results suggest that enhanced antigen presentation by cDC2s in the presence of high exposure to MHC-II peptide antigens supports the formation of cytotoxic Tr1 cells, which kill DC1s with granzyme B and do not produce IL-2, limiting cytotoxic CD8+ T cell-mediated antitumor immunity. However, the inhibitory effects of Tr1 cells could be overcome by targeting LILRB4 or CTLA-4, restoring IL-2, or eliminating cDC2s. Better understanding and targeting this mechanism could be used to improve antitumor immunotherapies moving forward.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Hussein Sultan answered our questions.
What was the most surprising finding of this study for you?
This project was full of unexpected discoveries. Initially, we observed that vaccines containing high, normally used, doses of a synthetic long peptide containing an MHC-II-restricted neoantigen surprisingly decreased the efficacy of various immune checkpoint therapies, such as anti-PD-1 and anti-4-1BB. This was particularly puzzling since CD4+ T cell responses are known to boost the antitumor efficiency of cancer vaccines, a concept supported by numerous studies from our lab and others worldwide. The second surprise was that vaccines containing high doses of MHC-II neoantigens didn’t generate conventional Foxp3+ regulatory T cells. Contrary to our expectations, these vaccines elicited suppressive Tr1 cells (Foxp3-negative non-conventional type 1 regulatory CD4+ T cells). A third surprise was that low doses of the MHC-II neoantigens (in the nanogram range) could still be effective and trigger helper CD4+ T cell response that could enhance the efficacy of cytolytic CD8+ T cells and effectively eliminate established tumors.
What is the outlook?
We hope these findings draw more attention to the critical role of type 1 regulatory T cells in diminishing the antitumor efficacy of cancer immunotherapies in patients. These findings could catalyze further investigations to identify the optimal vaccination strategies or platforms that can effectively stimulate robust effector antitumor T cell immunity while mitigating the induction of Tr1. We hope these findings will pave the way for more precise and effective cancer immunotherapies.
What was the coolest thing you’ve learned (about) recently outside of work?
I am astonished by the remarkable and ingenious design of the Gateway Arch in St. Louis, Missouri, or simply the St. Louis Arch. I was especially stunned by its method of construction. It was built from 1963 to 1965 using a method in which both legs were constructed simultaneously, and they had to meet at the exact center at the top. The margin of error was incredibly tight, and any misalignment could have been catastrophic. Remarkably, when the two legs finally joined, they were within 1/64th of an inch of being perfectly aligned. It is the tallest arch in the world, standing at 630 feet, and was built to withstand earthquakes and high winds.




