Despite the promise of immune checkpoint blockade (ICB) therapy in treating non-small cell lung cancer (NSCLC), the majority of patients do not respond and toxicity remains a major issue. Identifying biomarkers of ICB efficacy could direct treatment to responsive patient populations, but remains an ongoing challenge in the cancer immunotherapy field. Now, reported in Clinical Cancer Research, Hummelink et al. pinpoint a predictive biomarker for effective ICB response in NSCLC.
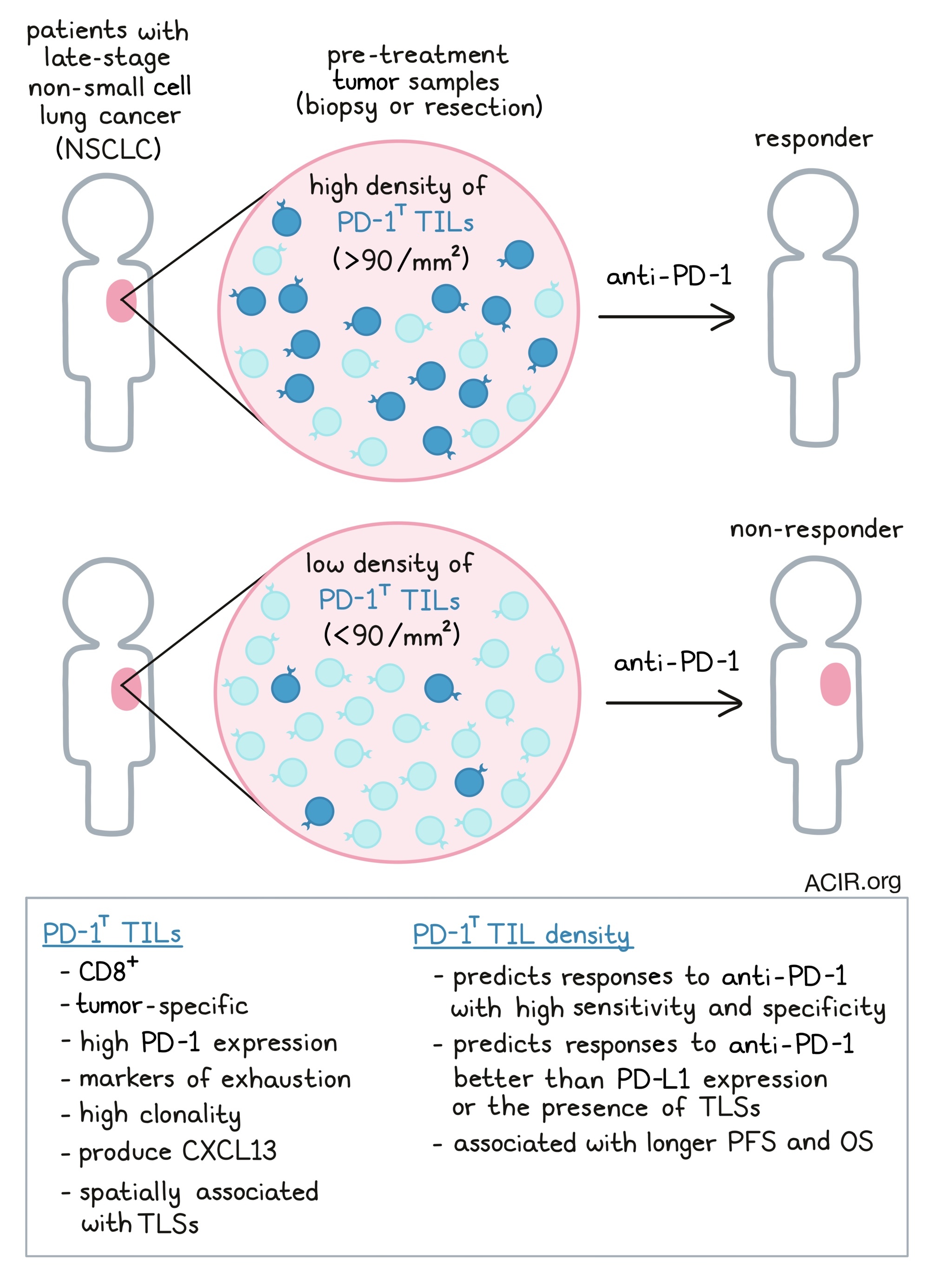
Previously, the group identified a unique tumor-infiltrating CD8+ T cell population with high PD-1 expression, dubbed PD-1T TILs (Thommen Nature Medicine 2018 [1]). These cells expressed markers of exhaustion but were highly clonal with tumor recognition capacity. Notably, their presence in pretreatment biopsies correlated with anti-PD-1 treatment response. In the current work, the team assessed whether this cell population possessed predictive capacity as a bona fide biomarker for ICB response.
To begin, Hummelink et al. collected baseline tumor specimens from two cohorts of late-stage NSCLC patients who later were treated with anti-PD-1 ICB (nivolumab or pembrolizumab). Formalin-fixed, paraffin-embedded tissue samples were stained and imaged using a digital, automated platform for PD-1T TIL quantification. The researchers segmented patients into training (n = 43) and validation (n = 77) cohorts, and tested the relationship between pre-treatment PD-1T TIL density (cells/mm2 in the section) and treatment response (disease control after 6 months). In the training group, PD-1T TIL density, specifically above or below 90 PD-1T TILs/mm2, predicted 6-month disease control with 79% sensitivity and 83% specificity. These findings were replicated in the validation cohort, in which PD-1T TIL levels also effectively predicted patient outcomes. Here, the PD-1T TIL density was >3-fold greater in ICB-responsive patients, and sensitivity and specificity of this biomarker were again high (77% and 67%). Importantly, across cohorts the team found that low PD-1T TIL density in pretreatment tumor specimens successfully indicated ICB non-responders.
Next, the researchers considered whether PD-1T TIL density could serve as a biomarker for additional treatment outcomes, such as 12-month disease control and survival metrics. Excitingly, disease control at 12 months was also predicted by PD-1T TIL levels (AUC = 0.78-0.89 and 92-93% sensitivity). In fact, the vast majority (97+%) of patients controlling disease at 12 months stratified in the high density (>90 PD-1T TILs/mm2) group. Progression-free and overall survival were also significantly higher in patients with high baseline PD-1T TIL density, compared to PD-1T-low patients.
ICB can lead to variable responses across tumor lesions in the same patient, and patients who experience progression of any lesion, even if others regress, are included in the progressive disease (PD) label based on RECIST criteria. Hence, the team next evaluated lesion-specific progression and PD-1T TIL density. This lesion-level characterization provided insight into local responses. Interestingly, among a set of 25 PD (12 month) patients with twice-measured CT responses, PD-1T-high biopsied lesions largely did not progress (~27%) compared to PD-1T low lesions (71%) in PD patients. Thus, local levels of PD-1T TILs may be predictive of ICB response on an individual lesion basis.
The authors next tested whether variables associated with tumor pathology or sample collection could influence the predictive ability of PD-1T TIL density. Promisingly, PD-1T TIL density was relatively consistent when sampling from different sites across a tumor, suggesting that even a small sample area could inform a tumor’s overall PD-1T TIL level. Further, clinicopathological features (such as adeno/squamous pathology, KRAS mutation status) or sample site (primary or metastatic) did not significantly alter PD-1T TIL density. However, the predictive value of PD-1T TILs was enhanced when samples were collected immediately prior to initiation of ICB, as opposed to before a previous line of therapy, and in tumor resection samples, versus biopsies.
Because PD-L1 levels have previously been used as a biomarker for ICB response, Hummelink et al. next compared the predictive capacities of PD-L1 expression and PD-1T TIL density. The PD-1T TIL metric (>90 PD-1T TILs/mm2) better predicted 6 and 12-month disease control compared to PD-L1 levels (either ≥50% or ≥1% expression). Additionally, adding PD-L1 expression analysis to PD-1T TIL density as dual biomarkers did not improve predictive ability over PD-1T TIL density alone.
Finally, the researchers assessed whether the presence of tertiary lymphoid structures (TLS) could indicate treatment outcomes. Previously, the team found that PD-1T TILs were associated spatially with TLS, and in fact secreted CXCL13, a B cell chemoattractant involved in TLS formation. Based on CD3 and CD20 co-staining density, the authors identified TLS (> 60,000 dul cells per µm2) in ~1/3 of tumors, and TLS along with lower density lymphoid aggregates (LA; 10,000 - 60,000 cells per µm2) in about 1/2. As expected, PD-1T TIL density was greater within TLS relative to non-TLS tumor tissue, and overall PD-1T TIL numbers were reduced in tumor sections lacking TLS, highlighting their association. However, TLS were found relatively consistently between PD-1T-high and low tumors, and PD-1T-high tumors had more PD-1T TILs both inside and outside TLS. Overall, PD-1T TIL density was more effective than TLS presence as a biomarker for ICB response.
Taken together, the researchers found that patients with low baseline PD-1T TIL density largely do not respond to anti-PD-1 therapy, highlighting a population for which ICB may not be an effective treatment. Identifying key populations of responders or non-responders may reduce overtreatment, avoiding substantial cost and serious side effects. Moving forward, further investigation of PD-1T TILs in different tumor types and mechanistic study of their role in the ICB response may support immunotherapy discovery and tailored application for more effective cancer treatment.
[1] Thommen, D.S., Koelzer, V.H., Herzig, P. et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 24, 994–1004 (2018). https://doi.org/10.1038/s41591...
Write-up by Alex Najibi, image by Lauren Hitchings
Meet the researcher
This week, first author Karlijn Hummelink and lead author Daniela Thommen answered our questions.

What was the most surprising finding of this study for you?
As PD-1 blockade therapy is only effective in approximately 30% of advanced lung cancer patients, predictive biomarkers are urgently needed to improve clinical treatment decision making. Therefore, we wanted to identify a biomarker that allows us to better select the patients who respond to this therapy. Surprisingly, we found that, whereas PD-1T TILs could distinguish patients with long-term benefit to PD-1 blockade, they were even better in very accurately identifying a patient group without any benefit. Therefore, this biomarker should allow for reduce overtreatment and toxicities while minimizing undertreatment.
What is the outlook?
Our retrospective study established PD-1T TILs as a novel predictive biomarker for durable clinical benefit to PD-1 blockade in lung cancer patients. Next steps would include validating this biomarker in larger prospective trials as well as in other cancer types. Furthermore, we would like to explore methods that could improve standardization across centers and can be routinely used in any hospital. For example, we are currently working on the development of a gene expression signature that measures the presence of PD-1T TILs in a tumor and that could be easily screened for each patient in a diagnostic biopsy.
What was the coolest thing you’ve learned (about) recently outside of work?
KH: Recently I visited Norway for the first time and experienced the beautiful fjords, waterfalls, and hiking trails. For me it was the perfect place to relax and clear my mind after my first year as a pathology resident. I would definitely recommend to go hiking and camping in this impressive landscape to fully recharge your battery.
DT: I recently visited the historical wine cellar of the Würzburg Residence and learned about their legendary wine from 1540. This wine resulted from an unusually hot year and was considered the best wine in a millennium. It is also the oldest existing wine; the second to last bottle was opened in 1961 after 421 years and was still drinkable! (at least the first two sips…). Now only one last bottle of this legendary wine is left.




