In a recent paper published in Nature, Chen et al. set out to elucidate the details of how PD-L1 upregulation by the tumor, and the subsequent immune evasion, predicts patient response, and how treatment efficacy could be improved. Building on prior data indicating that extracellular vesicles contain bioactive molecules, including PD-L1, the researchers began by isolating exosomes from human primary and metastatic melanoma cell lines and found that PD-L1 was indeed present in these exosomes, that the extracellular domain was exposed on the surface (recapitulating the topology of PD-L1 on tumor cell surface), that levels were increased following IFNγ treatment of tumor cells, and that the presence and levels of PD-L1 in exosomes were significantly higher in metastatic compared to primary melanomas. Similar findings were observed in the murine B16F10 metastatic melanoma cell line.
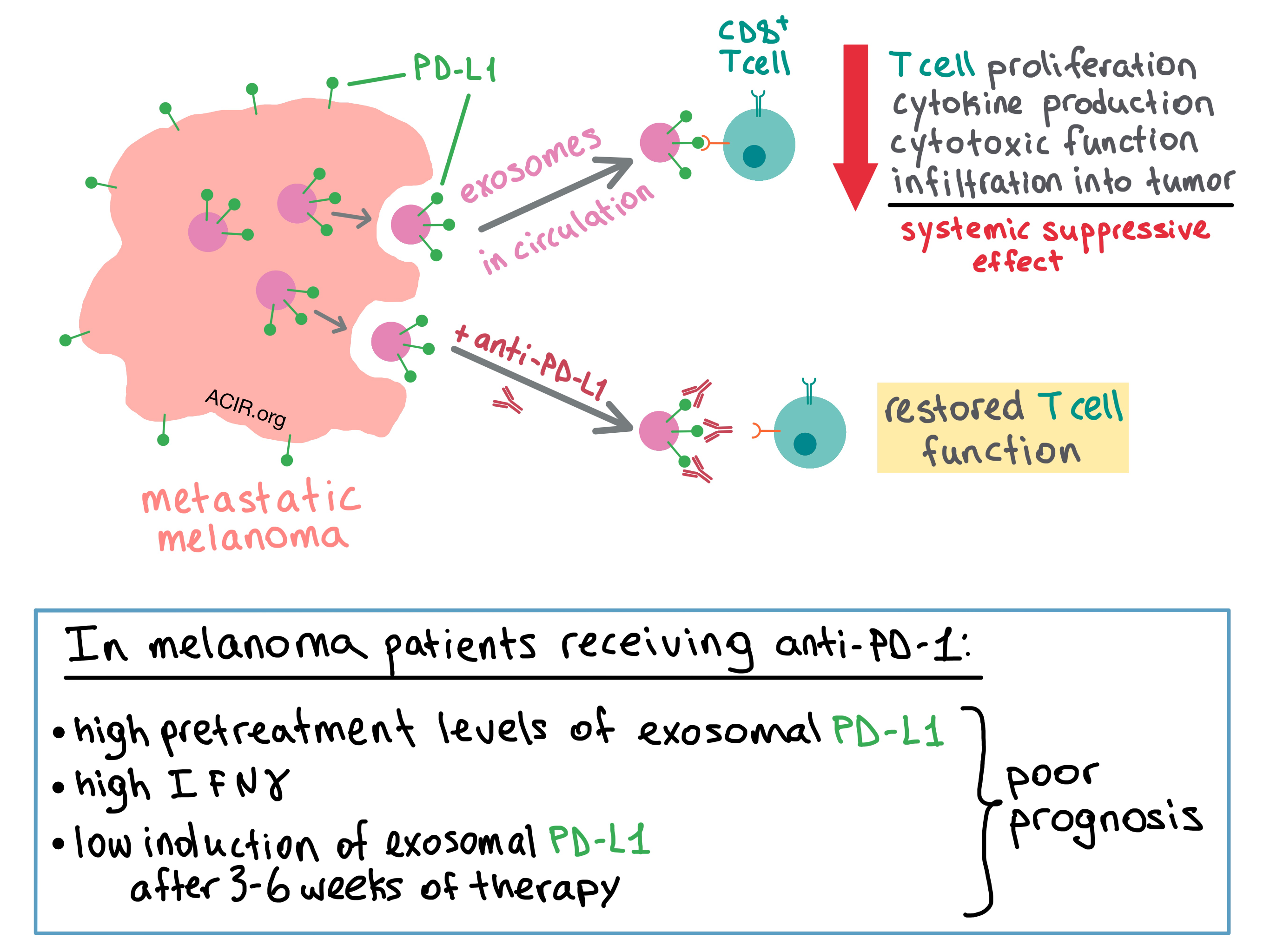
To examine the behavior of the exosomes in vivo, the team utilized a human melanoma xenograft model in nude mice. They isolated exosomes from the blood of the mice and found that the level of circulating exosomal PD-L1 positively correlated with tumor size. Next, the researchers utilized a syngeneic mouse model with a variant of B16F10 in which PD-L1 expression was knocked down; this abrogated the inhibitory effect of tumor-derived PD-L1 on antitumor T cells and slowed tumor growth. Injection of PD-L1-expressing exosomes re-established the inhibitory effect and promoted tumor growth of the B16F10 variant, while pretreating the exosomes with anti-PD-L1 antibodies reduced this effect. The presence of exosomes reduced the infiltration of CD8+ T cells into the tumor, as well as the proportion of proliferating PD-1+CD8+ T cells in the spleen and lymph nodes, suggesting a systemic suppressive effect on antitumor immune response.
The researchers then sought to find out whether exosomal PD-L1 inhibits CD8+ T cells. Chen et al. first used confocal microscopy to demonstrate that the melanoma-derived exosomes and T cells physically interact with each other, and then confirmed with flow cytometry that the interaction was stronger for activated versus non-activated CD8+ T cells. Treating the exosome-producing cells with IFNγ further increased exosomal binding to the T cells. PD-L1-expressing exosomes inhibited proliferation, cytokine production (IFNγ, IL-2, TNF), and cytotoxic function (granzyme B) of CD8+ T cells. These effects were abrogated when the exosomes were pretreated with anti-PD-L1 antibodies.
Convinced of the biological impact of PD-L1-containing exosomes, the team turned their attention to the exosomal PD-L1 levels in melanoma patients. While the number of circulating exosomes was similar between healthy controls and melanoma patients, the level of exosomal PD-L1 was significantly higher in the latter. The researchers then analyzed the PD-L1 expression on circulating exosomes in melanoma patients during anti-PD-1 therapy (pembrolizumab). The pretreatment level of exosomal PD-L1 was significantly higher in non-responders than in responders, and the elevated pretreatment level was associated with lower objective response rate (ORR). Non-responders also had higher pretreatment levels of IFNγ, which may have contributed to upregulated exosomal PD-L1. Overall, the level of circulating exosomal PD-L1 positively correlated with IFNγ levels and tumor burden, both of which indicated poor prognosis.
Although responders had lower baseline levels of exosomal PD-L1, the increase in the expression of PD-L1 during therapy (which was apparent at 3 weeks and peaked at 6 weeks in responders) was more enhanced in responders than in non-responders. In fact, a fold change of 2.43 in exosomal PD-L1 at 3-6 weeks of treatment stratified patient clinical response, with fold change >2.43 being associated with better overall response rate, progression-free survival, and overall survival.
Together, the results of this study suggest that melanoma cells systemically undermine the antitumor response by releasing PD-L1-expressing exosomes into the tumor microenvironment and circulation, where the exosomal PD-L1 binds with PD-1 on CD8+ T cells, suppressing their function. Disrupting the binding between exosomal PD-L1 and CD8+ T cells via anti-PD-1 or anti-PD-L1 antibodies may be one of the previously unknown mechanisms of actions of checkpoint blockade therapy. The authors suggest that circulating exosomal PD-L1 may serve as a blood-based biomarker and predictor of clinical response to anti-PD-1 therapy.
by Anna Scherer




