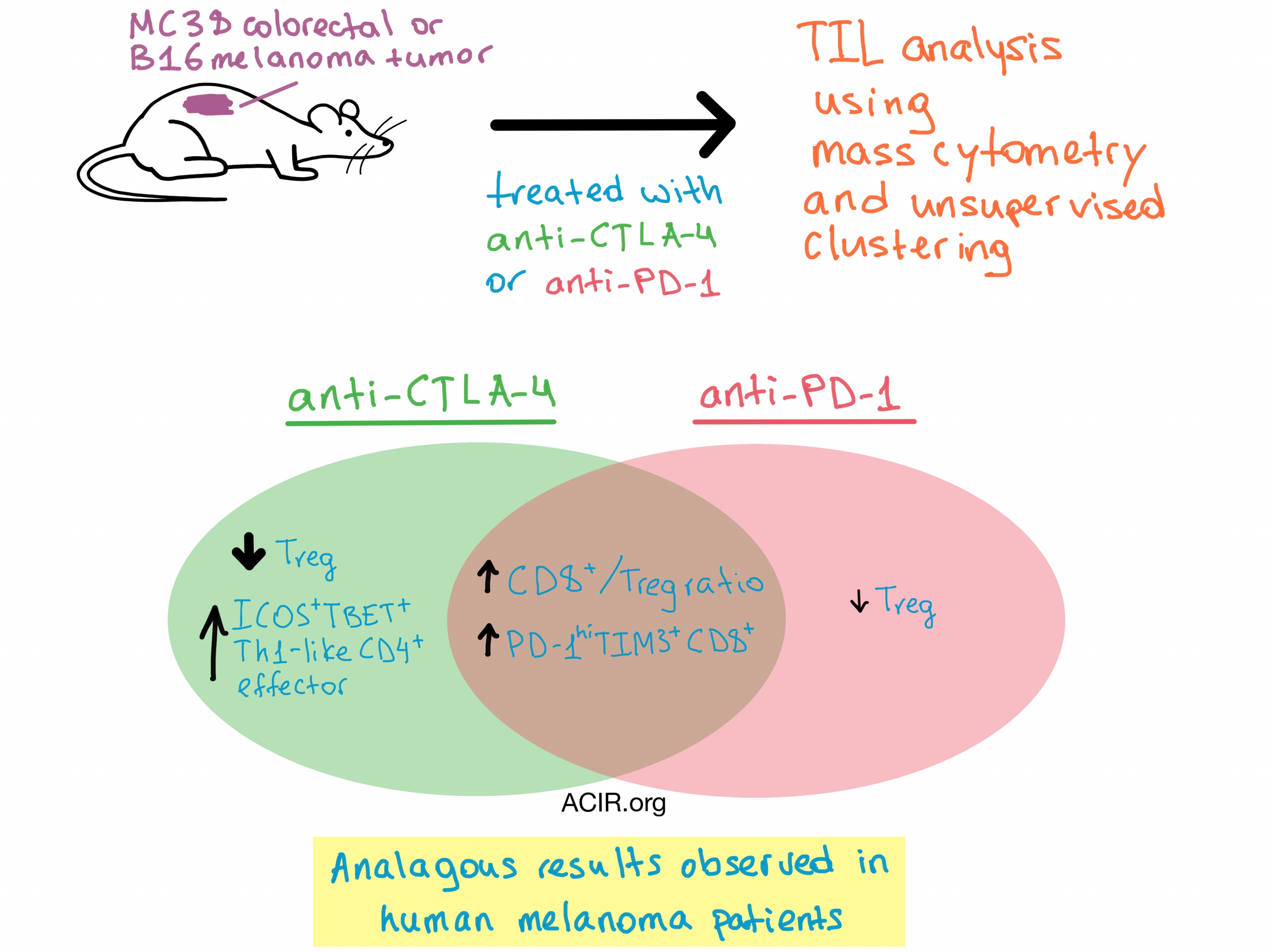
As the role of anti-PD-1 and anti-CTLA-4 immune checkpoint blockades (ICB) in cancer therapy continues to expand, understanding the underlying mechanisms of action becomes more imperative. Based on temporal, spatial, and cellular source differences, Wei et al. hypothesized that the anti-tumor immune responses to anti-CTLA-4 and anti-PD-1 treatment are driven by distinct mechanisms.
High dimensional mass cytometry is a powerful tool to interrogate multiple surface and intracellular markers on cells simultaneously, and coupled with unsupervised clustering analysis, it is capable of readily identifying cellular populations. To find out what goes on during the immune response following treatment, Wei et al. utilized this approach to identify and characterize the immune cells infiltrating tumors after ICB. The team transplanted syngeneic tumors into mice, which were then treated with either anti-CTLA-4 or anti-PD-1. The researchers specifically chose a tumor dose, treatment schedule, and analysis timepoint that resulted in incomplete rejection of tumors so that the tumor-infiltrating lymphocytes (TILs) could be analyzed.
In the highly immunogenic MC38 mouse colorectal tumor model, the team found an increase in the CD8+/Treg ratio after either treatment. Unsupervised clustering allowed for a deeper analysis of the data that revealed 15 distinct T cell clusters within the tumor. A phenotypically exhausted PD-1hiTIM3+CD8+ T cell population expanded more than other CD8+ T cell populations with either therapy. Based on short term labeling studies, this was likely due to increased proliferation within the tumor microenvironment, rather than increased T cell infiltration. The frequency of Treg cells decreased after both treatments, although, unsurprisingly, the effect was more pronounced following anti-CTLA-4 treatment. Finally, a significant increase in TBET+ Th1-like CD4+ effector cells was observed following treatment with anti-CTLA-4 but not anti-PD-1. A detailed look at the gene expression of MC38 tumor infiltrating cells revealed that ICOS+CD4+ T cells underwent significant, but mostly non-overlapping, transcriptional changes in response to anti-CTLA-4 and anti-PD-1 treatments, supporting the hypothesis that the two therapies have distinct underlying mechanisms.
The TIL analysis yielded nearly identical results in the B16/BL6 mouse melanoma model, an intrinsically weakly immunogenic tumor which required a GVAX tumor vaccine to boost overall T cell infiltration prior to ICB administration. Combined, the data from the two different mouse models suggest that the cellular mechanisms of both ICBs are different from one another, primarily with regard to CD4+ effector cell expansion, and are independent of tumor type.
To better understand the role of each tumor-infiltrating T cell subpopulation in the anti-tumor response, Wei et al. explored the correlations between T cell populations and tumor growth within the B16/BL6 model, and the results are presented below.
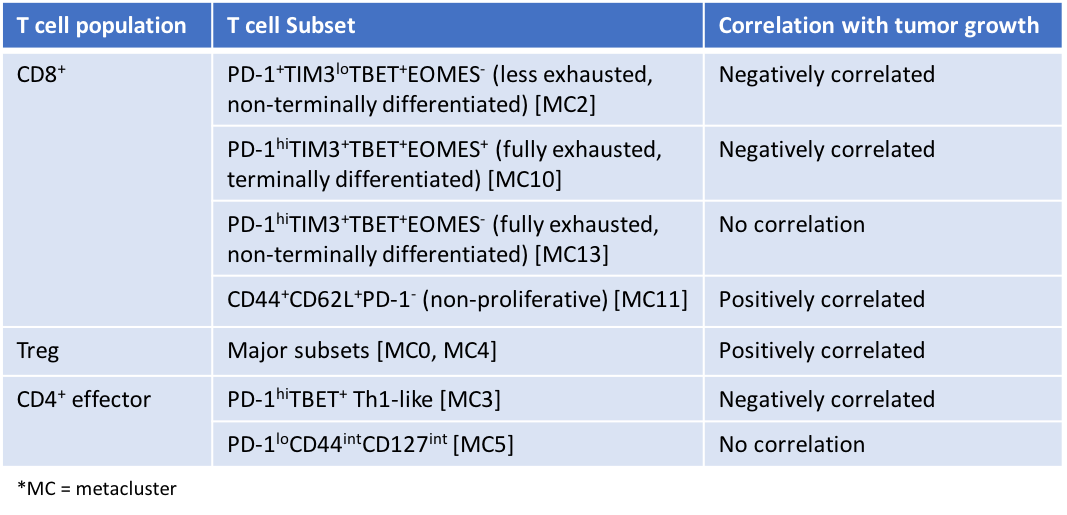
The two CD8+ T cell populations that were negatively correlated with tumor growth (MC2 and MC10) were activated and expanded after either ICB treatment. Surprisingly, one CD8+ phenotype (MC11) was positively correlated with tumor growth. This population was non-proliferative and may represent T cells with a central memory phenotype that are unrelated to the tumor, suggesting that infiltration of excess tumor-irrelevant cells may inhibit the anti-tumor response. Further analysis showed that the high proliferative rate of CD8+ T cells within the tumor in the later stages of the response was not necessarily correlated with anti-tumor effect. Overall, this detailed analysis indicates that only certain subpopulations of tumor-infiltrating T cells are involved in the response to ICB, and they may serve as predictive markers in the clinical setting. However, due to the study design of partial tumor regression, it is unclear whether the same mechanisms and cell populations would be observed during complete tumor regression, or whether the identified tumor-infiltrating T cell subsets are both necessary and sufficient for tumor rejection. In addition, the question remains whether all or only some distinct subsets of the phenotypically exhausted T cells that expanded following ICB treatment are functionally revived by ICB.
In a translational study, the researchers also performed mass cytometry analysis on resected tumors from 7 patients with metastatic melanoma who were treated with anti-PD-1, anti-CTLA-4, or a combination of both. They discovered that in the human setting, only two T cell subsets were significantly expanded in ICB-treated tumors compared to normal donor blood: the CD45RO+PD-1+TBET+EOMES+CD8+ T cell phenotype (exhausted, terminally differentiated), and the CD45RO+ICOS+PD-1loTBET+ Th1-like CD4+ effector T cell phenotype. These T cell populations were analogous to the corresponding phenotypes in the murine models (MC10 and MC3, respectively). The frequency of the Th1-like CD4+ effector T cells in human melanomas was higher after anti-CTLA-4 treatment compared to anti-PD-1 treatment, which was consistent with preclinical data.
Together, these murine and human results demonstrate that the mechanisms of anti-PD-1 and anti-CTLA-4 blockade are both similar (in the shared expansion of exhausted CD8+ populations by both therapies) and distinct (in the unique expansion of Th1-like CD4+ T cells by anti-CTLA-4 only), and contribute new insights and hypotheses to the optimization of combination therapies in the future.
by Anna Scherer




