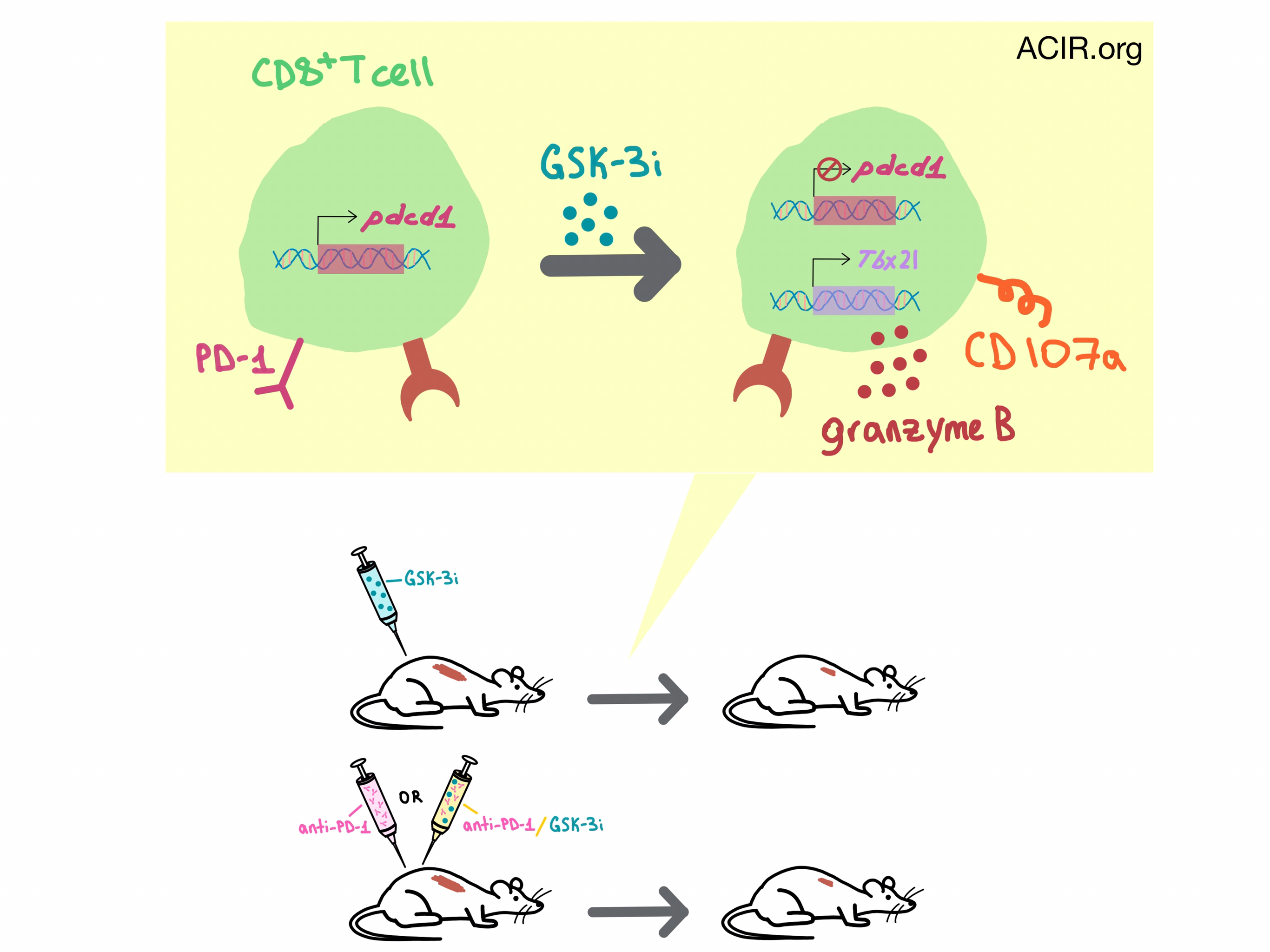
As anti-PD-1 antibody therapy can be costly and carries a risk of an autoimmune reaction, Taylor et al. explored the possibility of using a glycogen synthase kinase GSK-3 inhibitor (GSK-3i) as a small molecule inhibitor to downregulate PD-1 in the treatment of cancer.
In a recently published paper in Cancer Research, the team began with a study of metastatic tumors: they intravenously injected C57/BL6 mice with B16 melanoma tumor cells, and then treated the animals with either GSK-3i (SB415286) or anti-PD-1 every 2 days. After 14 days, the researchers analyzed the metastases in the lungs, and found that GSK-3i and anti-PD-1 showed similar levels of tumor inhibition, while combining the two therapies provided no further benefit. Similar results were observed in tumors that were allowed to establish for 7 days prior to treatment. To test for effects in primary solid tumors, the researchers intradermally injected mice with B16 cells, and then treated the animals with either GSK-3i, anti-PD-1, or GSK-3i and anti-PD-1 via intraperitoneal injections. All treatments increased survival to a similar degree.
Taylor et al. also assessed the effect of GSK-3i in an EL4 lymphoma model. EL4 cells pretreated with various concentrations of the SIINFEKL peptide of OVA were injected into the right flank, while cells that had not been pretreated were injected into the left flank of OT-1 Tg mice. Some of the mice were then treated with GSK-3i beginning on day 0 via a peritoneal injection. The team found that the growth of OVA peptide-pulsed tumor was reduced, but still visible in all animals not treated with GSK-3i. In contrast, administration of GSK-3i completely cleared the peptide-pulsed tumor mass in >80% of mice in 4 out of 7 experiments. The team also compared the efficacy of GSK-3i with anti-PD-1 and combination therapy, and found similar results across the board. Notably, at the highest tested OVA concentration (10 μg), all three treatments completely protected mice against death. In addition, similar effects on tumor growth were observed with anti-PD-L1 therapy.
The team also explored the effects of GSK-3i vs. anti-PD-1 on EL4 tumors in the absence of the OVA peptide. They found that tumors completely regressed in some mice treated with either anti-PD-1 or combination therapy, but not GSK-3i alone. Meanwhile, combination therapy improved survival over either individual therapy.
To understand how GSK-3i exerts its effect on the tumors, Taylor et al. utilized quantitative RT-PCR and flow cytometry to study its mechanism of action. They found that GSK-3i reduced pdcd1 (PD-1) and increased Tbx21 (Tbet) transcription in CD8+ T cells, and increased the percentage of tumor-infiltrating CD8+ T that expressed CD107a and granzyme B. No PD-1 downregulation was observed on CD4+ T cells or Treg cells. In genetic studies, tumor growth reduction in GSK3α/β-/- knockout mice was similar to Pdcd1-/- mice, and the addition of anti-PD-1 to GSK3α/β-/- mice or GSK-3i to Pdcd1-/- mice resulted in no further tumor reduction. GSK-3i had no direct effect on the tumor. Altogether, the data indicates that GSK-3i limits tumor growth by downregulating PD-1 expression on CD8+ T cells.
Given this proposed mechanism of action, the team explored the effect of GSK-3i in an adoptive transfer setting. Mice were intradermally injected with EL4-OVA cells, and 7 days later they underwent adoptive transfer with OT-1 cytotoxic T cells that had been cultured in vitro for 7 days in the presence or absence of OVA peptide plus GSK-3i, anti-PD-1, or a combination of the two. T cells treated only with OVA delayed the onset of tumor growth at all peptide concentrations. Pretreatment with either GSK-3i, anti-PD-1, or a combination of the two further slowed tumor growth at all peptide concentrations, and completely cleared the tumor at the highest peptide concentration. The downregulation of PD-1 expression by a single dose of GSK-3i persisted for 7-10 days.
To find out whether GSK-3i has a similar effect on human cells, Taylor et al. isolated human CD3+CD8+ T cells from peripheral blood and demonstrated that GSK-3i also downregulates PD-1 expression to a similar extent in this cell population.
The cumulative results of these experiments demonstrate that the GSK-3i effect on tumor growth is mediated by its effect on the downregulation of PD-1 and upregulation of Tbet expression in CD8+ T cells, and its efficacy is similar to anti-PD-1 therapy, indicating that GSK-3 inhibitors, which have been clinically tested in type II diabetes and neurological disorders, could serve as a potential small molecule alternative to anti-PD-1 antibody in cancer therapy.
by Anna Scherer




