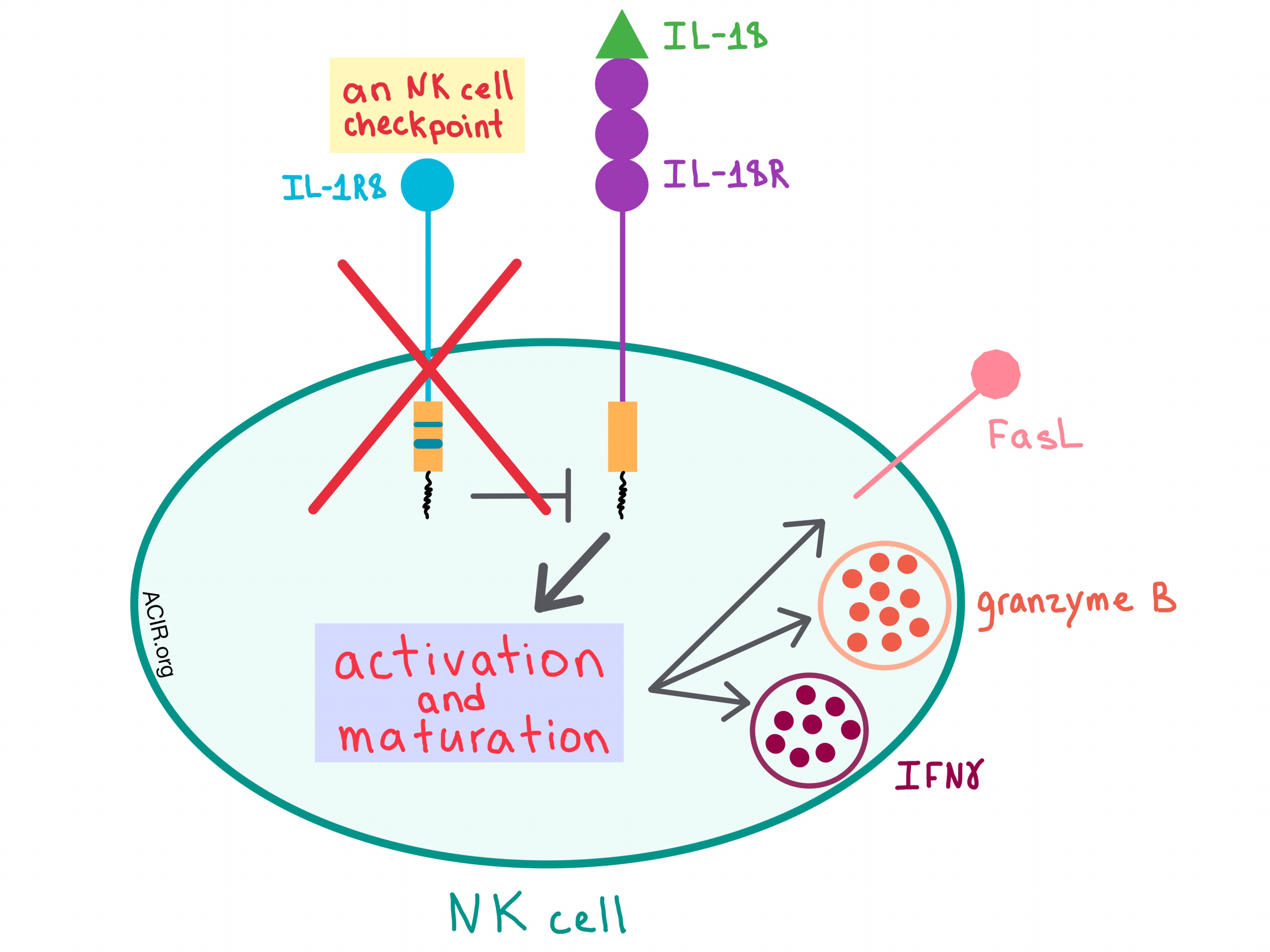
In a recent paper published in Nature, Molgora et al. demonstrated that interleukin-1 receptor 8 (IL-1R8), which is a negative regulator of the IL-1 receptor (ILR) and Toll-like receptor (TLR) pathways, acts as a checkpoint for natural killer (NK) cell maturation and function, and removing this checkpoint could affect tumorigenesis and metastasis.
Although many cells express IL-1R8, Molgora et al. found that among both human and mouse immune cells, it is particularly abundant on NK cells. As human NK cells mature, the mRNA levels and protein expression of IL-1R8 increases, and this pattern is recapitulated in mouse NK cells, thus allowing the researchers to examine the effects of this receptor in mouse models.
To understand how IL-1R8 acts on NK cells, Molgora et al. utilized IL-1R8-deficient (Il1r8-/-) mice, and found that the knockout of IL-1R8 resulted in increased NK cell frequency and count and a more mature NK cell phenotype. NK cells within the peripheral blood of these mice had an upregulated expression of activating receptors, and, when stimulated ex vivo with IL-18, the cells increased the production of IFNγ and granzyme B and expression of FasL, indicating that IL-1R8 influences NK cell function.
Since IL-18 is involved in NK cell differentiation and function, the researchers sought to further elucidate its relationship with the IL-1R8 pathways. Removing the effect of IL-18 either genetically or via an antibody blockade in Il1r8-/- mice abrogated enhanced NK cell maturation and function, suggesting that IL-1R8 regulates the IL-18 signaling pathways in NK cells. To better understand the mechanism of action of IL-1R8, the researchers performed RNA sequencing, which revealed that the transcriptional changes in NK cells due to IL-1R8 deficiency involved activation pathways (such as MAPK), adhesion molecules (such as ICAM-1), and increased production of some chemokines (such as CCL4).
To confirm that IL-1R8 also acts as a checkpoint in humans, Molgora et al. treated normal human NK cells with a combination of IL-18 and IL-12, and observed an inverse correlation between IL-1R8 levels and IFNγ production. In addition, partial silencing of IL-1R8 with small interfering RNA resulted in increased IFNγ production, thus indicating that, like in mouse cells, IL-1R8 acts as a negative regulator of NK cell activation in humans.
Having confirmed that the relationship between IL-1R8 and NK cell maturation and function is similar between humans and mice, the researchers went on to test the effect of IL-1R8-mediated regulation of NK cells in a tumor setting in several mouse models. Because the liver has a high concentration of NK cells, the team started with a model of hepatocellular chemical carcinogenesis. Compared with Il1r8+/+ mice, fewer tumor lesions that were also smaller in size developed in the Il1r8-/- mice, and within these lesions, there were more NK cells, higher levels of antitumor cytokines (eg, IFNγ), and lower levels of pro-inflammatory cytokines. Consistent with other studies suggesting that NK cells may have a beneficial role in preventing blood-borne metastases, similar results were observed in a model of sarcoma spontaneous lung metastasis, where Il1r8-/- mice had fewer metastases, although the primary tumors were unaffected. In both mouse models, the protection against cancer development or spread was NK cell-dependent, as depletion of the NK cells (but not CD4+ or CD8+ T cells) abrogated the protective effect. Il1r8-/- mice were also protected against MC38 colon carcinoma liver metastasis, and genetically knocking out IL-18 negated this protection, once again confirming that IL-1R8 acts through the IL-18/IL-18R axis. Further, adoptive transfer of Il1r8-/- NK cells into tumor-bearing Il1r8+/+ mice reduced the number and volume of liver and lung metastases, while adoptively transferred Il1r8+/+ NK cells had no effect.
As the effects of an immune checkpoint blockade can often go both ways, IL-1R8-deficient mice experience deregulated activation by ILR or TLR ligands, which leads to increased inflammation and, in turn, the development of certain cancers and autoimmune disease. The differential effect of IL-1R8 deficiency on carcinogenesis may reflect the fundamental differences in tissues at different anatomic sites, including the level of NK cell infiltration. Therefore, NK cells have the potential to affect solid tumors and metastases if the appropriate checkpoints, such as IL-1R8, are blocked and the tissue-specific immunological landscape is taken into consideration.
by Anna Scherer




