Flt3L (FL) is essential for the differentiation and proliferation of classical DCs (cDCs), which play a role in recruiting various immune cell types and activating both effector and regulatory responses in cancer. Investigating the role of FL in antitumor immunity, Régnier et al. uncovered an unusual pattern, in which both high and low expression of FL improved antitumor immunity. Investigating this pattern, they untangled a web of relationships between FL, cDCs, Tregs, effector T cells, and NK cells, and developed strategies to improve antitumor immunity. Their results will be published in Cell Reports Medicine.
To begin, Régnier et al. injected mice with B16 melanoma tumors, and collected draining lymph nodes at different time points over the course of tumor development. Using multiparameter flow cytometry, they found that resident (r) rDC1s, rDC2s, plasmacytoid (p) pDCs, and inflammatory monocytes were transiently increased early, peaking at day 5. Tregs and NKs mirrored the increase in DCs, while macrophages increased later.
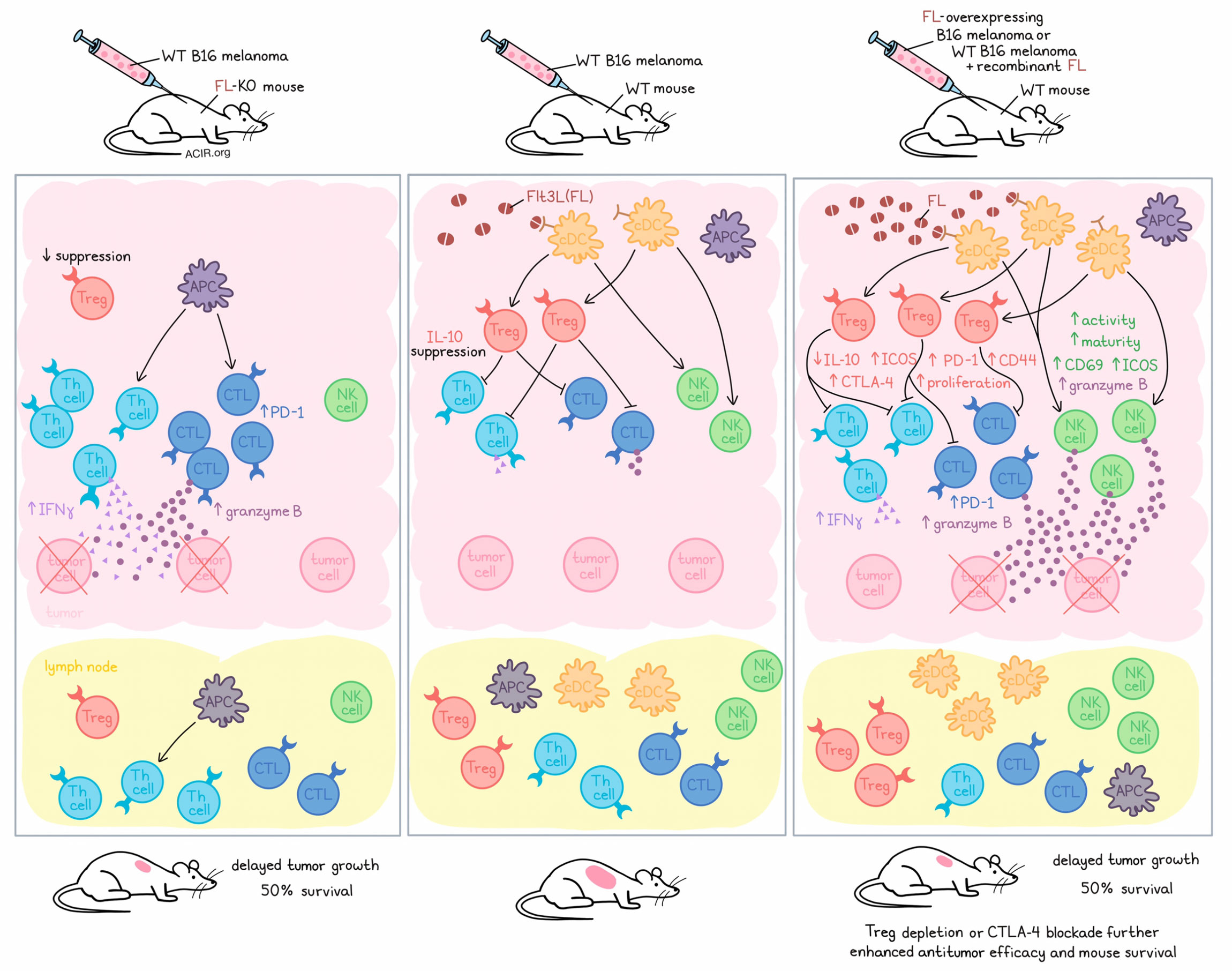
To evaluate the effects of DCs in tumors, the researchers evaluated FL deficiency (using FL-knockout mice bearing WT B16 melanoma) and FL overexpression (using WT mice bearing FL-overexpressing B16 melanoma or WT mice with WT B16 melanoma treated with recombinant FL during tumor development). Interestingly, both reduced and enhanced FL expression delayed tumor growth and extended survival in mice. Similar results were observed in the YUMM1.7 melanoma model, but not in MC38 tumors, which are immunologically “hot”.
Looking more closely at how FL levels alter the immune environment in their cold tumor models, the researchers showed that FL levels were positively associated with both transient and sustained increases in the proportions of various cDC subsets in dLNs, and negatively associated with Langerhans cells and inflammatory monocytes. FL levels also positively associated with Tregs expressing increased in CD44, CTLA-4, and PD-1 in both tumors and lymph nodes. However, both FL deficiency and FL overexpression were associated with reduced IL-10 production by Tregs, FL overexpression was associated with increased expression of ICOS. Further, while FL was negatively associated with Th cells in dLNs, Th cells were increased in both FL-deficient and FL-overexpressing (or soluble FL-treated) tumors (compared to wild-type), along with their expression of IFNγ. Similarly, cytotoxic T lymphocytes (CTLs) and their expression of PD-1 and of granzyme B were increased in tumors in both FL-deficient and FL-overexpressing tumors.
The link between FL expression levels and the presence of activated Tregs was found to be determined by levels of FL-dependent DCs (mainly cDCs) mediating enhanced Treg proliferation, while the relationship between FL-dependent DCs and activated Th cells was limited or negative. Further, antitumor efficacy was dependent on CD4+ and CD8+ T cells in FL-deficient tumors, but not in FL-overexpressing tumors. Together, these results suggest that FL deficiency improves antitumor immunity by depleting cDCs, and in turn, reducing the accumulation of Tregs and their suppressive effects on effector CD4+ and CD8+ T cells.
Investigating how FL overexpression improves antitumor immunity, Régnier et al. found that FL was positively associated with bona fide NK cells expressing increased levels of CD69, ICOS, and granzyme B, and with NK maturation in tumors. Further, levels of NK cells in the lymph nodes also correlated with levels of FL-dependent DCs. As Flt3 was not expressed on NK cells, the researchers concluded that FL increased DCs, which in turn, increased NK cells and NK cell activity. This was further confirmed in a setting of both FL overexpression and DC deficiency in tumors. Similar patterns were observed in mice lacking tumors, suggesting cDC-mediated control of NK cell homeostasis. Depletion experiments showed that these changes in NK cells contributed to antitumor immunity in FL-overexpressing tumors, with additional contributions from CD8+ T cells.
Moving towards strategies to enhance antitumor immunity, Régnier et al. showed that both FL overexpression and Treg depletion showed similar antitumor efficacy, but that the combination of the two further improved survival, with mice surviving over 100 days – 5 times longer than untreated mice. Anti-CTLA-4 also enhanced the antitumor efficacy of FL overexpression, but anti-PD-1 did not.
To determine whether these results were relevant in human cancer, the researchers analyzed transcriptomic data from The Cancer Genome Atlas (TCGA) melanoma cancer (SKCM) project and classified groups of patients based on low, medium, or high expression of FL mRNA. Here, as in mice, FL was positively associated with DC1s, DC2s, pDCs, NK cells, and Tregs. DC1/DC2 gene signatures also strongly correlated with Treg and NK signatures. Further, IL15, IL15RA, and (to a lesser extent) IL2 expression were correlated with DC1, DC2, and NK cell signatures, suggesting that, like in mice, DCs support NK cell homeostasis through trans-presentation of IL-15.
The paradoxical pattern in which survival was improved with high or low FL expression was observed in three cancer types: acute myeloid leukemia (AML), Wilms tumor, and pancreatic cancer. High FL expression was associated with beneficial outcomes in 17 cancer types, and with detrimental effects in 7 cancer types. Gene signatures for DC1, DC2, and pDCs showed similar patterns, mirroring FL expression. Overall, FL expression and/or DC infiltration was related to survival in 35 of 42 evaluated human cancers.
Finally, in cancers where FL expression had a paradoxical effect, either high FL expression or a low Treg signature was associated with better survival outcomes. Survival was further enhanced in patients who had both high FL expression and a low Treg signature, with 70% of those patients surviving longer than 5 years.
Overall, these results show that FL supports cDCs, which mediate both effector and regulatory immune responses in tumors. In some tumor types, an interesting pattern was observed in which either FL deficiency or FL overexpression enhanced antitumor immunity. On one hand, FL deficiency mediated adaptive antitumor immunity by reducing DCs, which limited the accumulation of Tregs and allowed for effector CD4+ and CD8+ T cell-mediated antitumor immunity. On the other hand, FL overexpression supported DCs, which supported an influx of mature NK cells into tumors, which unleashed innate antitumor immunity. Understanding these mechanisms could be useful in the development of cancer immunotherapies, as anti-CTLA-4 and Treg depletion synergized with FL modulation, and similar paradoxical patterns involving FL were identified in some human cancers.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Paul Régnier and lead author Guillaume Darrasse-Jèze answered our questions.

What was the most surprising finding of this study for you?
When we decided to evaluate the role of dendritic cells (DCs) in the immune response to melanoma in mice, we were genuinely astounded to discover that both their deficit or excess, paradoxically, extended the life of tumor-bearing mice. Then, the big challenge was to understand the biological mechanism behind the paradox. We found that the homeostatic control of Natural Killer (NK) cells and regulatory T cells (Tregs) by dendritic cells was really the linchpin explaining these phenomena. The next major revelation was that this paradox extends to certain human cancer types as well, and that tumor-infiltrating dendritic cells could also prove purely beneficial or detrimental in other types of cancer.
What is the outlook?
These results really change our perspective on the immune response in cancer and have important implications for improving immunotherapy. But the road is long, as we would like to understand if precise subsets among Flt3L-dependent DCs are responsible for NK and/or Treg recruitment during cancer immune responses. Furthermore, we seek to identify which antigen-presenting cells are capable of priming T cells and unleashing the antitumor adaptive immune response in the absence of Flt3L-dependent DCs. We hope to leverage this knowledge to improve immunotherapy protocols, particularly in cancers where Flt3L-dependent DCs have detrimental impacts on patient responses to treatment.
What was the coolest thing you’ve learned (about) recently outside of work?
Both the first author, Paul Régnier, and I recently shared the experience of being fathers to young children. This has proven to be an additional daily source of joy, surprises, and discovery!




