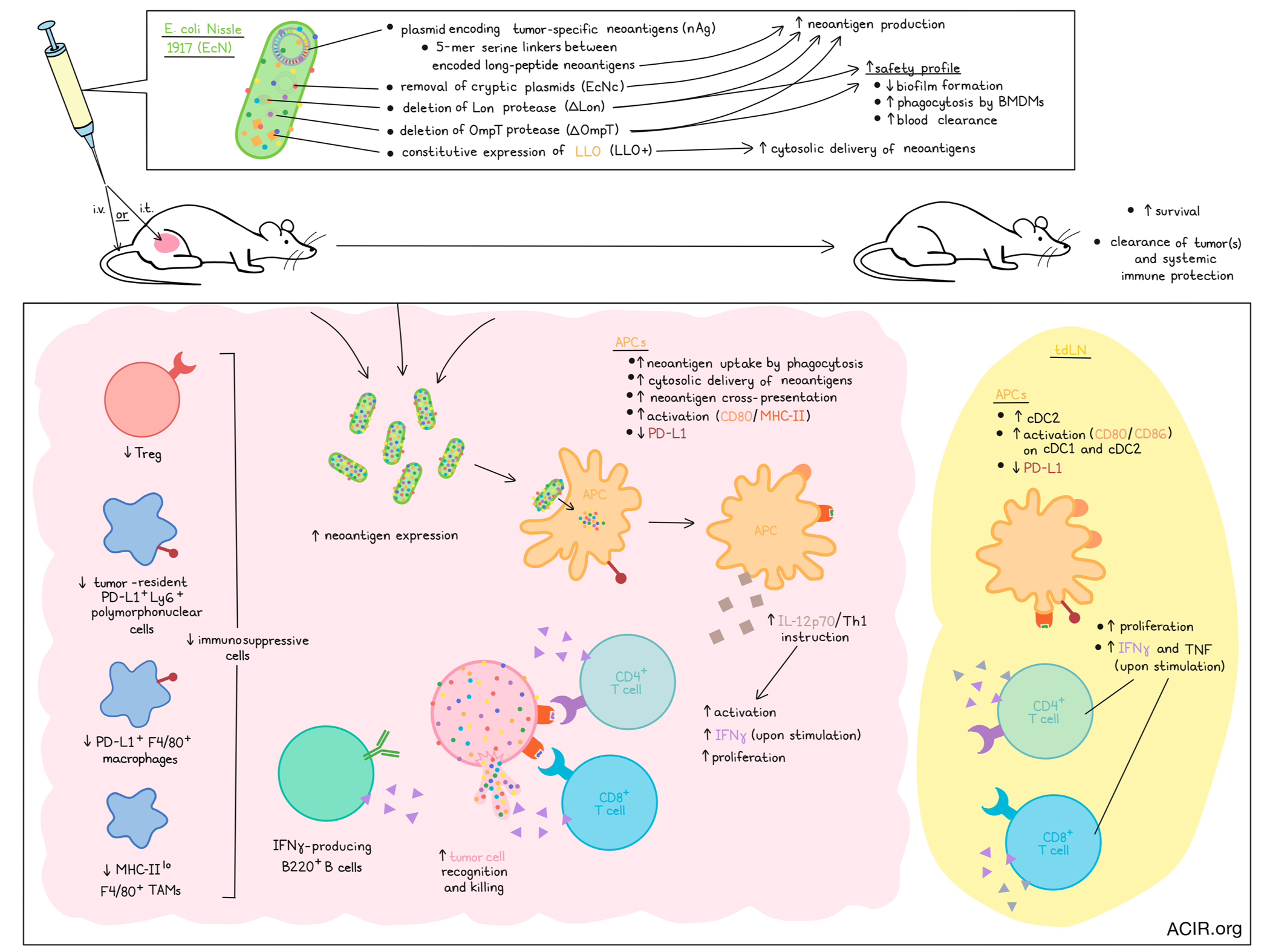
In cancer immunotherapy, vaccination with tumor-specific neoantigens can be an effective way to induce antitumor immunity. In recent work, Redenti and Im et al. developed a novel system to deliver neoantigens using an engineered probiotic E. coli Nissle 1917 (EcN) – a bacterial platform that inherently homes to tumors, stimulates immune responses, and inhibits immunosuppressive mechanisms. Their results describing the development and optimization of this platform to induce antitumor immunity were recently published in Nature.
To begin, Redenti and Im et al. sequenced CT26 colorectal tumors and predicted immunogenic neoantigens based on highly expressed tumor-specific mutations and predicted binding to MHC-I and MHC-II. They then created a prototype gene encoding a synthetic neoantigen construct of linked long peptides, cloned that gene into a stabilized plasmid, and transformed EcN cells, which then expressed the encoded peptides at low levels.
To increase the expression of neoantigens by transforming EcN, the researchers incorporated 5-mer glycine-serine linkers between neoantigen long peptides in the prototype gene, increasing neoantigen production 6-fold. Next, they removed the EcN cryptic plasmids, leading to roughly 30-fold higher levels of therapeutic plasmid DNA (EcNc). Finally, deletion of both the Lon protease (EcNcΔlon) and OmpT protease (EcNcΔompT) enabled up to 80-fold increased production of encoded neoantigens. These modifications also left the probiotic products attenuated in biofilm formation, more susceptible to phagocytosis by bone marrow-derived macrophages (BMDMs), and more sensitive to blood clearance, enhancing the safety profile. Using a gene construct encoding OVA as a model antigen, the researchers showed that EcNcΔlon/ΔompT-OVA, but not EcN-OVA, increased neoantigen uptake and cross-presentation by APCs, enhanced APC activation (MHC-II and CD80), and reduced APC PD-L1. Finally, the researchers induced constitutive expression of LLO – a pH-dependent pore-forming protein that permeabilizes phagolysosomal membranes, enabling cytosolic delivery of neoantigens – which led to increased BDMC secretion of IL-12p70 (indicating greater Th1 instruction by APCs) and mediated enhanced activation of naive OT-I and OT-II T cells, which secreted higher levels of IFNγ and IL-2 and were more proliferative.
To test the efficacy of the EcNcΔlon/ΔompT/LLO+ platform in vivo, mice with CT26 tumors subcutaneously transplanted in a hind flank were treated with an intratumoral (i.t.) injection of EcNcΔlon/ΔompT/LLO+ carrying either a single neoantigen construct, or a combination of 3 neoantigen constructs (nAg19; each with unique neoantigens from the predicted set, selected along a spectrum of predicted affinity for MHC class I and MHC class II). Treatment with EcNcΔlon/ΔompT/LLO+ nAg19 induced strong tumor control and extended survival compared to controls, and was well tolerated. The inclusion of the LLO expression was shown to contribute significantly to the observed antitumor effects, and led to increased expression of IL-12p70, enhancing Th1 instruction.
Next, Redenti and Im et al. investigated EcNcΔlon/ΔompT/LLO+ nAg19 across various tumor and delivery settings. In mice with CT26 tumors on both hind flanks, i.t. treatment of one tumor induced regression of tumors on both sides in several animals. With no evidence of bacterial migration to the untreated tumors, these results suggested induction of systemic antitumor immunity. Intravenous (i.v.) injection of EcNcΔlon/ΔompT/LLO+ nAg19 in mice with advanced CT26 tumors also induced potent antitumor efficacy and extended survival, outperforming standard vaccination with synthetic long peptides, with no evidence of toxicity. The engineered microbes were found at high densities within tumors, while they were readily cleared from all other organs that were evaluated. EcNcΔlon/ΔompT/LLO+ nAg19 was also effective in a model of metastasis (intravenous CT26 engraftment), where the microbial platform colonized metastases-bearing lungs (but not other tissues), restrained metastatic tumor growth, and extended survival compared to other treatment groups. Additionally, prophylactic vaccination of naive mice reduced growth of relevant tumors, and no tumor growth was observed upon rechallenge in mice that had previously cleared CT26 tumors after treatment, suggestive of immune memory.
After confirming that encoded neoantigens were effectively expressed in situ, the researchers noted that i.v. treatment of CT26 tumor-bearing mice with EcNcΔlon/ΔompT/LLO+ nAg19 increased cDC2s and increased activation (CD80+ and CD86+) of cDC1s and cDC2s in tdLNs. CD4+ and CD8+ T cells isolated from tdLNs at day 8 showed increased proliferation and production of IFNγ and TNF production upon stimulation with phorbol myristate acetate (PMA) and ionomycin, while TILs isolated at the same time point showed increased IFNγ production upon stimulation with a pool of synthetic peptides representing the 19 bacterially encoded tumor neoantigens. Looking at individual neoantigen responses, the researchers found that several MHC-I and MHC-II neoantigens from each construct were effectively targeted. Further, tumor-infiltrating CD4+ and CD8+ T cells showed increased proliferation, and ex vivo coculture of TILs with CT26 cells showed target cell recognition and killing. Restimulation with PMA/ionomycin increased IFNγ-producing CD4+ and CD8+ T cells (compared to stimulation with peptides), and increased IFNγ-producing B220+ B cells, suggesting epitope spreading and expanded immune activation. Further, treatment with EcNcΔlon/ΔompT/LLO+ nAg19 resulted in reduced frequencies of tumor-resident immunosuppressive PD-L1+Ly6G+ polymorphonuclear cells, PD-L1+F4/80+ macrophages, Tregs, and MHC-IIloF4/80+ TAMs in tumors. Treatment also reduced expression of PD-L1 on cDC1s and cDC2s in tdLNs, suggesting that EcNcΔlon/ΔompT/LLO+ nAg19 also targets several immunosuppressive tumor defenses.
To extend their results to additional models, Redenti and Im et al. developed a similar EcNcΔlon/ΔompT/LLO+ targeting 42 neoantigens (spread across seven constructs) derived from B16F10 melanoma. Like in CT26 models, i.t. or i.v. treatment of mice bearing orthotopic B16F10 tumors was well tolerated and led to colonization of tumors, reduced tumor growth, and extended survival, dependent on CD4+ and CD8+ T cells, which were also capable of direct tumor cell killing ex vivo. Treated tumors had increased cDC1s, cDC2s, conventional CD4+ T cells, cytotoxic CD8+ T cells, NK cells, and inflammatory monocytes. CD4+ and CD8+ T cells also showed enhanced activation, proliferation, and effector functions. Tregs, MDSCs, MHC-IIlo macrophages, and regulatory B cells (an important immunosuppressive cell type in B16F10) were also reduced, suggesting reduced immunosuppression, while expression of MHC-II on monocytes and DCs increased, suggesting increased antigen presentation. EcNcΔlon/ΔompT/LLO+ nAg42 was also effective in a model of established, systemic B16F10-Luc metastasis.
Overall, these results show that an engineered microbial system optimized for production and cytosolic delivery of tumor-specific neoantigens can induce strong systemic antitumor immunity and reduce tumor-mediated immunosuppression, leading to the tumor elimination, extended survival, and protection from tumors in mouse models. This vaccine delivery method also demonstrated features of enhanced safety and was well tolerated, paving the way for potential clinical applications.
Write-up and image by Lauren Hitchings




