In a recent paper published in Cell, Su, Zhao, and Xing et al. explored the effect of antibody-dependent cellular phagocytosis (ADCP) on tumors and unexpectedly discovered that following monoclonal antibody treatment, ADCP in macrophages led to suppression of natural killer (NK) and T cells as well as poor antitumor response in breast cancers and lymphomas.
To begin, the researchers fluorescently labeled HER2+ breast cancer cell lines (SKBR3 and BT-474) and monocyte-derived human macrophages with different dyes and co-cultured them either in the presence or absence of trastuzumab (a humanized anti-HER2 monoclonal antibody used in the treatment of breast and gastric cancers). Trastuzumab induced phagocytosis of HER2+ cancer cells by macrophages via Fc gamma receptors (FcγRs). In a series of experiments, the team was surprised to find that ADCP in macrophages inhibited the trastuzumab antibody-dependent cellular cytotoxicity (ADCC) by autologous NK cells and suppressed the proliferation and cytotoxicity of tumor-specific T cells. Similar results were observed with Raji CD20+ lymphoma cells and rituximab (an anti-CD20 monoclonal antibody used in the treatment of leukemia and lymphoma).
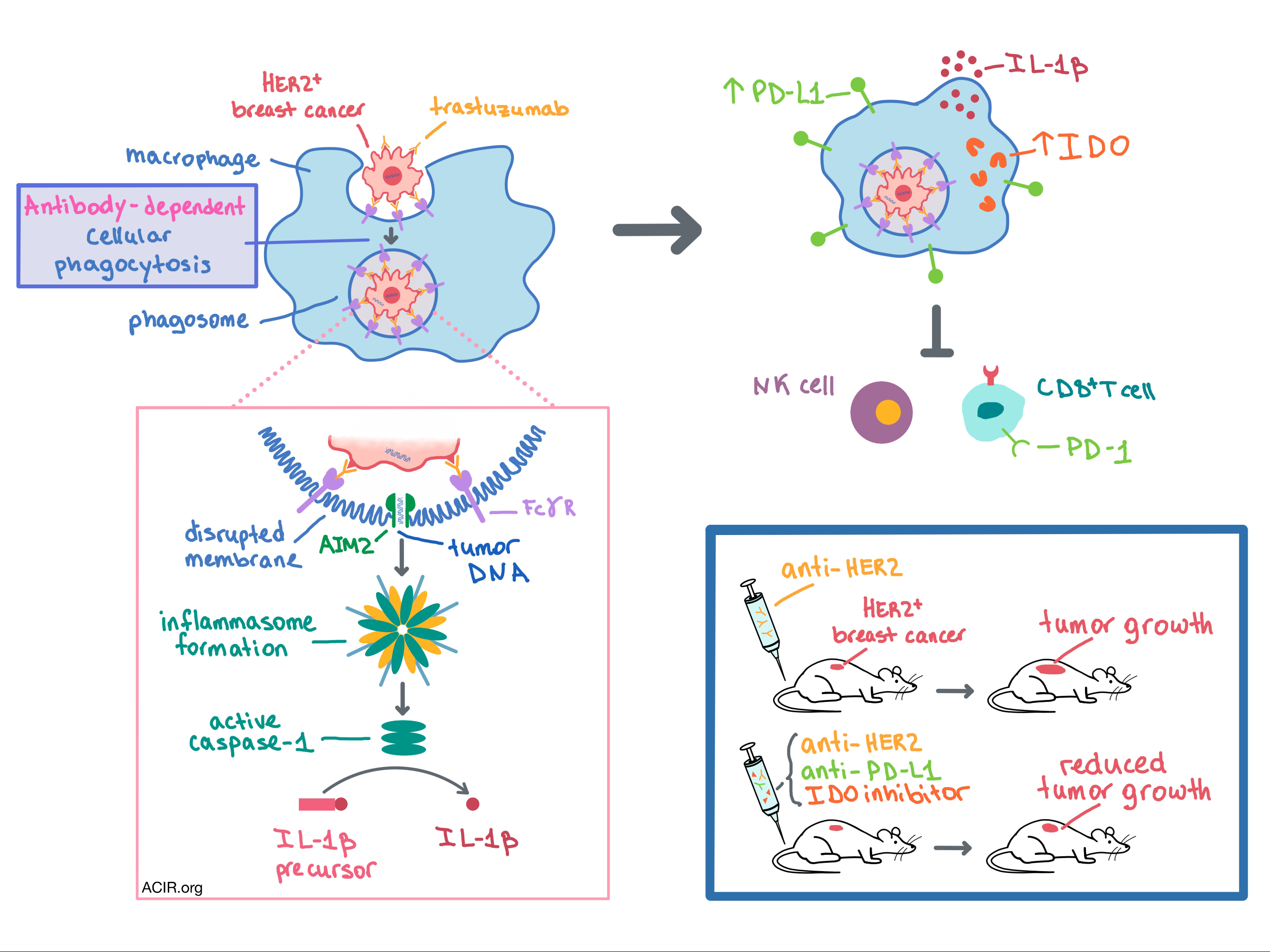
In order to explain the surprisingly immunosuppressive effect of ADCP in macrophages, the researchers dove deep into mechanistic studies. In their numerous experiments, the team utilized gene expression analysis, qRT-PCR, transcriptomic profiling, Western blot, immunohistochemistry, and confocal microscopy. Ultimately, the following mechanism emerged. After antibodies recognize and bind their target on the tumor cell, FcγRs on the surface of macrophages bind the Fc antibody fragments, initiating ADCP. Once the tumor cell-containing phagosome is inside the macrophage, FcγR signaling recruits AIM2 (a cytosolic double-stranded DNA sensor) to the membrane of the phagosome. The phagosomal membrane is disrupted, and AIM2 detects tumor DNA (both mitochondrial and genomic), leading to inflammasome formation. The inflammasome activates the caspase-1 enzyme, which cleaves the IL-1β precursor to create its bioactive, mature form. The active IL-1β is released, leading to the overexpression of PD-L1 and IDO in the macrophages that phagocytosed antibody-coated tumor cells as well as in the adjacent bystander macrophages. PD-L1 and IDO expression in macrophages then mediates immunosuppression of NK and T cells. The mechanism by which IL-1β, usually a proinflammatory cytokine, leads to the upregulation of PD-L1 and IDO in macrophages remains unclear.
Having observed that macrophage ADCP was responsible for the suppression of NK and T cells in vitro, the researchers sought to explore the effects of trastuzumab in vivo. To do this, Su, Zhao, and Xing et al. expressed human HER2 in the mouse E0771 breast cancer cell line and injected these cells into transgenic mice that are immune tolerant to human HER2. In one set of these mice, macrophages were genetically ablated. Mice were then treated with 4D5, a mouse anti-human HER2 antibody that is the parental drug to trastuzumab. In mice without macrophages, the treatment led to decreased tumor growth and increased infiltration of NK and CD8+ T cells in the tumor compared to mice with intact macrophages. In addition, the treatment increased the expression of PD-L1 and IDO in tumor-associated macrophages (TAMs). While adding anti-PD-L1 or an IDO inhibitor to 4D5 reduced tumor growth in mice with intact macrophages, combination of all three therapies synergized to boost the therapeutic effect even further. Mechanistically, the triple therapy dramatically increased infiltration of NK and CD8+ T cells in the tumor. These beneficial effects were not observed in mice without macrophages. The involvement of the AIM2/IL-1β pathway in suppression of the anti-HER2 antibody’s therapeutic effect was observed in vivo via knockout and blockade experiments.
The researchers then sought to confirm the clinical relevance of their pre-clinical observations. To that end, they analyzed resected primary tumors from HER2+ breast cancer patients who had received neoadjuvant trastuzumab prior to surgery. In non-responders, macrophages were more abundant while NK and CD8+ T cell infiltration was reduced compared with responders. Levels of PD-L1 and IDO in the pre-treatment tumor biopsies were low; however, PD-L1 and IDO expression increased significantly in TAMs, but not in tumor cells, following trastuzumab treatment. Higher post-treatment number of PD-L1+IDO+ TAMs was associated with poor response to trastuzumab.
Overall, Su, Zhao, and Xing et al. demonstrate, and mechanistically explain, that trastuzumab therapy in humans and mice with HER2+ breast cancer leads to upregulation of PD-L1 and IDO in TAMs via ADCP, which in turn inhibits the antitumor response of NK and CD8+ T cells. These results provide the rationale for combining immune checkpoint blockade with therapeutic antibody treatment to improve anti-cancer response.
by Anna Scherer




