Aiming to understand the requirements for successful immunotherapy outcome, Alspach et al. explored the role of CD4+ T cells in antitumor response and found that expression of MHC-II neoantigens in the tumor, activation of CD4+ T cells, and CD4+ T cell help for CD8+ T cell priming and maturation were critical for response to immune checkpoint blockade (ICB) and cancer vaccines. The results were recently published in Nature.
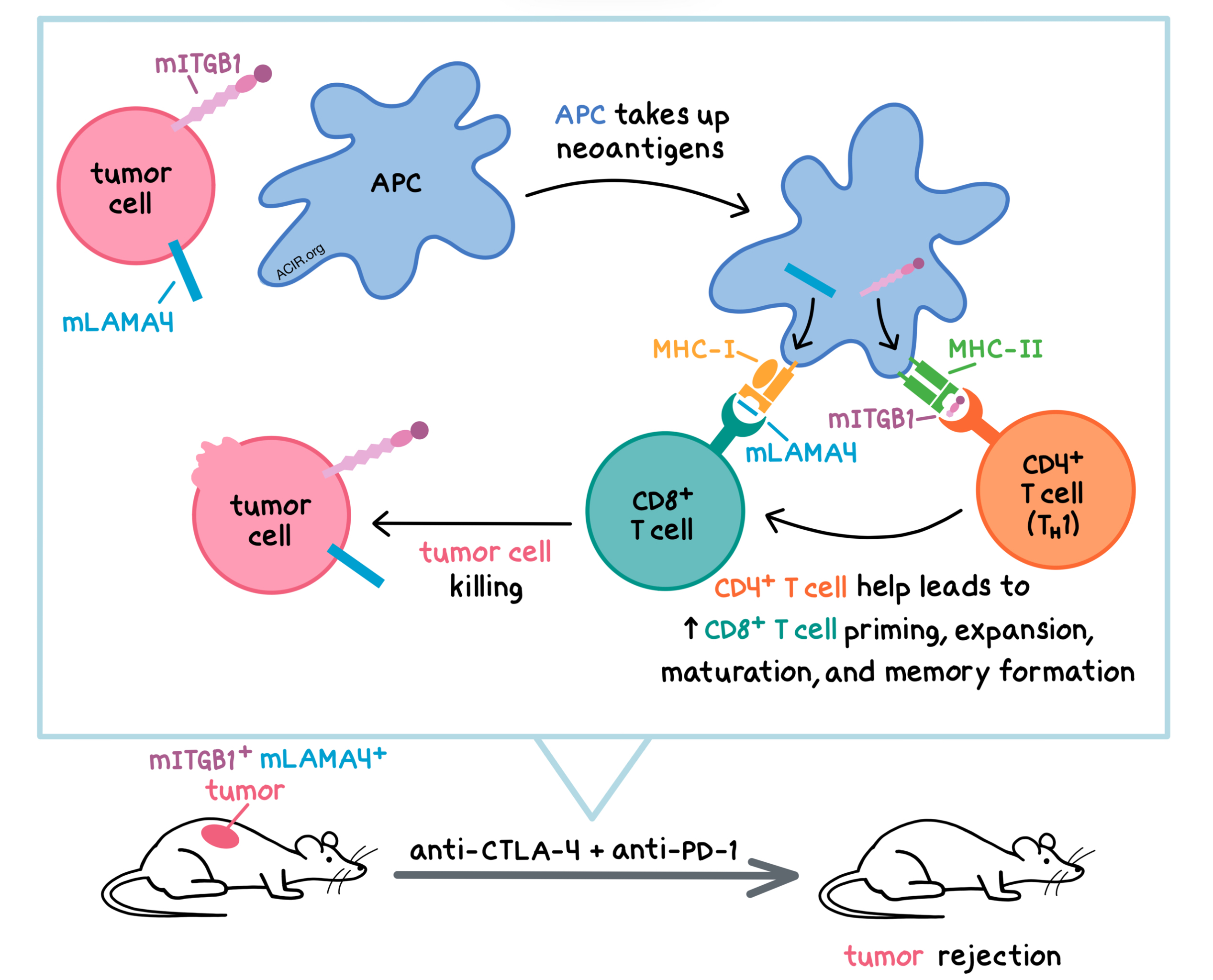
Given the limitations of the currently available MHC-II neoantigen prediction tools, the researchers began by developing a new prediction algorithm – a hidden Markov model-based MHC binding predictor (hmMHC) that takes into account variable length peptide sequences and is trained on the most recent Immune Epitope Database data. Using hmMHC, Alspach et al. analyzed 700 missense mutations expressed in the MHC-II-negative T3 sarcoma tumors and identified an N710Y somatic point mutation in integrin-β1 (mITGB1) as a neoantigen presented by the MHC-II I-Ab molecule. N710Y in ITGB1 is the second most highly expressed mutation in T3 tumors. This neoantigen was not predicted by the existing netMHCII-2.3 or netMHCIIpan-3.2 algorithms. In vitro, mITGB1 peptide induced T3 tumor-infiltrating CD4+ T cells to produce IFNγ, TNF, and IL-2, but not IL-4, IL-10, IL-17, or IL-22, suggesting that these mITGB1-specific CD4+ TILs had a TH1-like phenotype. No mITGB1-specific CD8+ T cells were found. Together, these experiments demonstrated that mITGB1 is an MHC-II-restricted neoantigen in T3 cancer.
Next, the researchers used the KP9025 (KP) sarcoma model, which contains only 4 mutations, none of which were predicted to be immunogenic, to explore whether CD4+ T cells were necessary for tumor rejection during ICB. To this end, they ectopically expressed the T3-derived MHC-I neoantigen (mLAMA4) and/or MHC-II (mITGB1) neoantigen in KP cells. The unmodified KP tumors grew progressively in wild-type mice and did not respond to the anti-PD-1 + anti-CTLA-4 combination, confirming that KP tumors were not immunogenic. Expression of either mLAMA4 or mITGB1 did not alter KP tumor immunogenicity. However, KP tumors expressing both neoantigens grew slower in wild-type mice and were rejected following either single agent or dual ICB. The antitumor effect was observed despite the fact that the tumor cells did not express MHC-II. Based on these observations, the antitumor response and sensitivity to ICB were dependent on both CD8+ and CD4+ T cells.
Alspach et al. then sought to identify the mechanism by which CD4+ T cells affect response to ICB. In KP.mLAMA4.mITGB1 tumors, mITGB1-specific CD4+ T cells exhibited an activated TH1 phenotype, similar to T3 tumors. Comparing splenic CD8+ T cells from mice with KP.mLAMA4 tumors versus mice with KP.mLAMA4.mITGB1 tumors, the latter had a larger percentage of mLAMA4-specific CD8+ T cells before and after ICB treatment, as measured by tetramer staining, and this percentage was further increased with anti-CTLA-4 (either as monotherapy or in combination with anti-PD-1). Furthermore, anti-CTLA-4 increased the ratio of T-bet+ (TH1) to Foxp3+ (Treg) tumor-infiltrating, mITGB1-specific CD4+ T cells. In an in vivo cytotoxicity assay, only mice with KP.mLAMA4.mITGB1 tumors could eliminate mLAMA4 peptide-pulsed splenocytes, and this was further enhanced by ICB. Together, these experiments demonstrate that CD4+ T cell help increases CD8+ T cell priming and facilitates their maturation into cytotoxic effectors.
Having shown that CD4+ T cells are important in ICB, the researchers wondered whether mITGB1-specific CD4+ T cells also play a role in vaccine-mediated antitumor response. While vaccination of naive mice with irradiated KP, KP.mLAMA4, or KP.mITGB1 cells did not protect the animals from subsequent T3 tumor challenge, combination vaccine of irradiated KP.mLAMA4 and KP.mITGB1 cells protected 30% of mice, and vaccination with dual-expressing irradiated KP.mLAMA4.mITGB1 cells conferred protection to 85% of mice. Consistent with this, there were more mLAMA4-specific, IFNγ+ CD8+ T cells in the spleens of mice vaccinated with KP.mLAMA4.mITGB1 cells than those vaccinated with KP.mLAMA4 cells. Thus, vaccines required both MHC-I and MHC-II neoantigens, preferably in the same tumor cell, for maximum antitumor efficacy.
To confirm that tumor cell expression of MHC-II neoantigens was required for tumor rejection, Alspach et al. injected wild-type or immunodeficient Rag2-/- mice with KP.mLAMA4 tumors on one flank and KP.mLAMA4.mITGB1 tumors on the opposite flank and then treated the animals with ICB (anti-PD-1 + anti-CTLA-4). In Rag2-/- mice, tumors grew at the same rate in both flanks, as expected. In wild-type mice, KP.mLAMA4.mITGB1 tumors were completely rejected while the growth of KP.mLAMA4 tumors was only delayed. Thus, tumor cell expression of both MHC-I and MHC-II neoantigens was necessary for mLAMA4-specific CD8+ T cell-mediated tumor rejection. Moreover, CD4+ T cell help and tumor cell expression of MHC-II neoantigens were required for the maintenance of functional CD8+ T cell memory to protect mice from tumor rechallenge.
The results of this study suggest that patients with immunogenic MHC-I neoantigens and high tumor mutation burdens may not respond to immunotherapy if they lack immunogenic MHC-II neoantigens. Development of reliable and accurate MHC-II neoantigen prediction algorithms could help identify potential responders in the clinic.
by Anna Scherer
Meet the researcher
First author Elise Alspach answered our 3 questions.

What prompted you to tackle this research question?
Cancer immunotherapies have revolutionized the ways in which patients are currently treated, and have provided viable options for conditions previously considered untreatable. However, immunotherapies are effective in only a minority of patients who receive them. We wanted to investigate the things that dictate immunotherapy success versus failure, and provide some insight that may one day make immunotherapy more widely successful.
What is the most surprising finding of this study for you?
I think we were most intrigued by the requirement for MHC-II neoantigen expression in the tumor. Other groups have suggested that CD4+ T cell help did not have to be tumor-specific, indicating that the antigen didn’t need to be expressed by the tumor cells themselves. Additionally, our tumor models don’t express MHC-II. But nevertheless, our results using the minimal neoantigen-based sarcoma cells clearly indicate that MHC-II neoantigen expression at the tumor site is needed for optimal tumor rejection after immunotherapy treatment. These results have important implications for patient selection, and may shed some light on what dictates how a patient will respond to immunotherapies.
What was the coolest thing you’ve learned recently outside of work?
It may not be the coolest thing I’ve learned, but the idea that it “takes a village” has definitely become very apparent recently. In both my science and during parenthood, I would not have been able to achieve the things that I have without the support and contributions of my family, coworkers, and collaborators. Nothing that is worth doing is done in a bubble.




