Dendritic cell (DC)-based cancer vaccines have great potential but are currently lacking in efficacy. Adamik et al. investigated the transcriptomic and immune-metabolic characteristics of DCs from patients with melanoma to better understand how cancer may impact antigen presentation function and metabolic activity of these cells. Their results were recently published in Nature Communications.
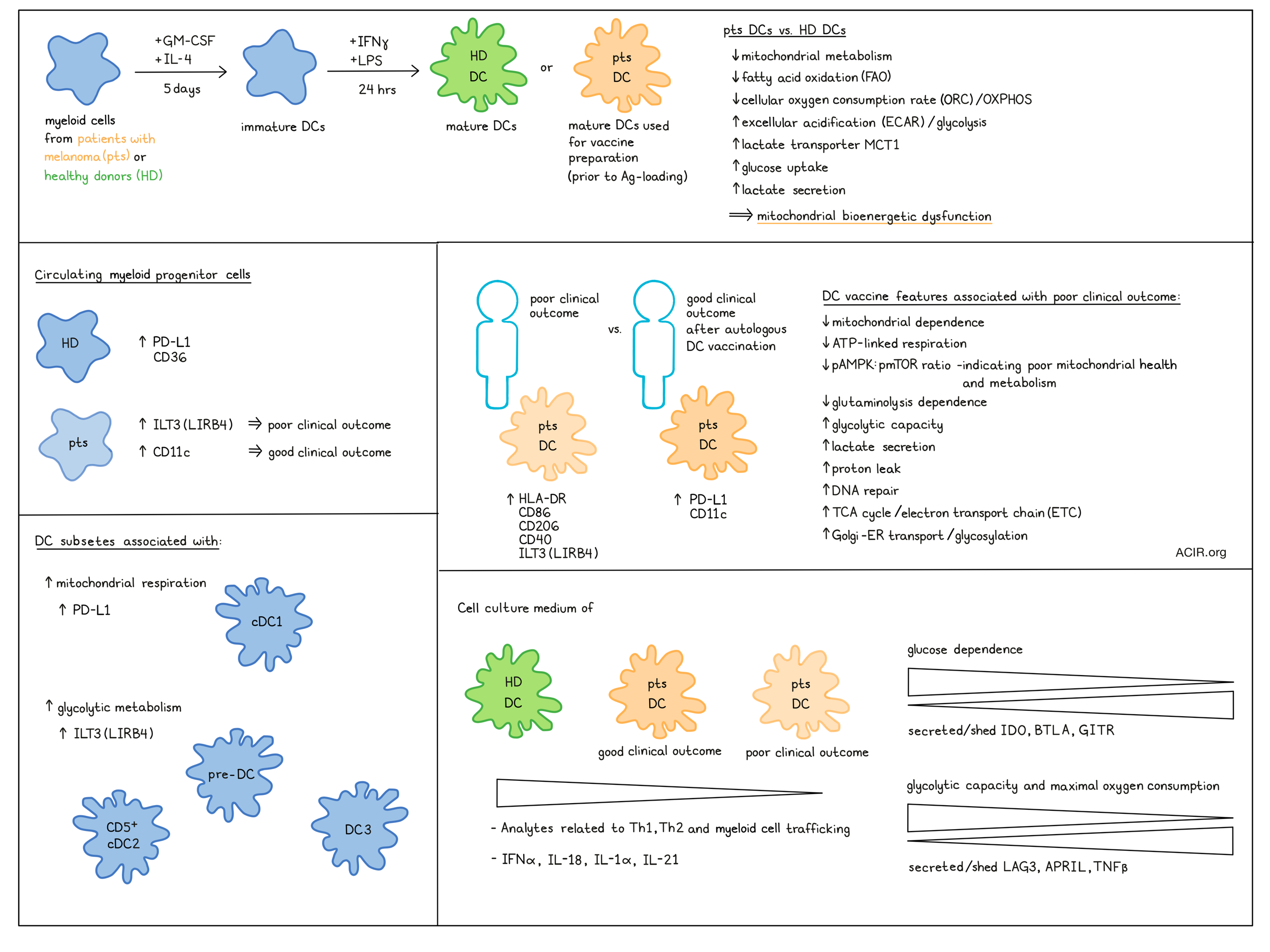
The researchers analyzed cells obtained from patients enrolled in a Phase I trial in late-stage melanoma testing antigen-engineered autologous DC vaccines. For the trial, peripheral blood myeloid cells were cultured for 5 days with GM-CSF and IL-4 (immature DCs; iDC), matured with IFNγ + LPS for 24 hours (mature DCs; mDC), and then loaded with antigen by adenoviral infection. For this metabolic study, transcriptomic and immune-metabolic profiling was performed on patient- or healthy donor (HD)-derived, IFNγ + LPS mDCs prior to adenoviral infection.
Microarray analysis of mDC showed upregulation of 725 and downregulation of 818 genes as compared to healthy donor (HD) mDC. There was a selective downregulation of metabolic genes involved in the TCA cycle and electron transport chain (ETC)/OOXPHOS in HD and fatty acid/phospholipid metabolism and PPAR pathway in melanoma mDC. There were also differences in IL-2, IL-3, and STAT3 signaling pathways in melanoma mDC. Comparing mDC from patients with good clinical outcomes (partial remission (PR) or stable disease (SD) >6 months and non-recurrent no evidence of disease (NED)) and bad clinical outcomes (progressive disease (PD) and SD ≥6 months and recurrent NED), revealed those with better outcomes were enriched in immune and metabolic pathways. On the other hand, genes related to DNA repair, TCA/ETC, mRNA processing, interferon signaling, and Golgi-ER transport/glycosylation were upregulated in those with poor outcomes. Furthermore, genes related to cytokine activity and extracellular matrix disassembly immunoregulatory genes were upregulated in good outcome mDC.
To confirm that these changes in metabolic gene expression profiles had downstream effects, mitochondrial and glycolytic metabolism was assessed. Melanoma mDC showed a trend toward lower maximum oxygen consumption rate (OCR) and spare respiratory capacity. There was a significant increase in basal glycolysis in patient mDC, and glycolytic capacity was mainly increased in the poor outcome group. Melanoma mDC had a reduced ability to metabolize long-chain fatty acids, and had an increase in proton leak, in particular in the poor outcome group. Furthermore, the poor outcome group mDC also had decreased ATP-linked respiration. These data align with the transcriptomic-based data suggesting mitochondrial bioenergetic dysfunction in melanoma mDC.
Using the single-cell energetic metabolism by profiling translation inhibition (SCENITH) assay, the impact of metabolic changes on the immune phenotype of the mDC was assessed. A decrease in mitochondrial dependence with a corresponding increase in glycolytic capacity and a decrease in glutaminolysis dependence was detected in mDC, in particular in the poor outcome group. Higher mitochondrial dependence was associated with a longer overall and progression-free survival (OS and PFS, respectively).
In poor outcome patient mDC, immune and costimulatory molecules (including HLA-DR, CD86, CD206, and CD40) were overexpressed, as well as the inhibitory checkpoint molecule ILT3 (aka LIRB4). Interestingly, the expression of these molecules did not significantly impact melanoma-specific T cell responses.
Treatment of cells with a mitochondrial inhibitor and monitoring the rates of protein synthesis can distinguish cells that demonstrate reduced protein synthesis and are mitochondrial-dependent and cannot use aerobic glycolysis vs. highly glycolytic cells that maintain protein synthesis. Compared with HD and good outcome mDC, poor outcome mDC had the highest number of cells in the highest glycolytic quantile. A higher ratio of p-AMPK:p-mTOR, which is an indicator of mitochondrial health and metabolism, was observed in cells with the highest mitochondrial dependence, consistent with more dependence seen in the good outcome mDC.
CyTOF-based single-cell analysis of metabolic molecules (scMEP) and pathway clustering allowed for visualization of patient iDC (day 5 of culture, before IFNγ and LPS addition) and mDC (Day 6 of culture)-specific metabolic regulome differences. There was no specific clustering related to clinical outcomes, but some of the good-outcome patients clustered together with HD mDC. In mDC, scMEP markers clustered cell populations with higher HLA-DR separate from those with higher CD11b and CD14 expression. CD86 and HLA-DR expression were downregulated over time in culture in melanoma-derived DC, particularly in the worse outcome group. PD-L1 was downregulated in patient mDC, but PD-L1 and CD11c were increased in DCs derived from patients who had melanoma-specific T cell responses.
The lactate transporter MCT1 (related to glycolytic metabolism) was increased in melanoma mDC, while the β-oxidation pathway enzyme HADHA was decreased, which is consistent with reduced fatty acid oxidation capacity. MCT1 expression was correlated with lactate levels in culture media from patient-derived DCs, and, as expected, lactate was inversely correlated with media glucose concentration. Increased lactate secretion by DC, but not decreased glucose concentration, by DC was correlated with worse OS.
Assessing culture media for cytokines, chemokines, growth factors, and secreted/released surface receptors, the researchers found that melanoma DC secreted lower levels of these analytes than HD. Analytes related to Th1, Th2, and myeloid cell trafficking were highest in HD, then good outcome, and lowest in poor outcome DC groups. IFNα, IL-18, IL-1α, and IL-21 were highly expressed in HD and good outcome, while reduced in poor outcome cultures. Glucose dependence of mDC was inversely related to the secretion/release of IDO, BTLA, and GITR, while LAG3, APRIL and TNFβ were inversely correlated to glycolytic capacity or maximal oxygen consumption of mDC.
Adamik et al. then assessed whether the circulating myeloid progenitor cells used to derive the DCs from patients were different from HD. The monocyte and plasmacytoid clusters of HD clustered separately from the patient cells, suggesting this may be the case. HD monocytes had higher expression of PD-L1 and CD36 than patient cells. In patients, increased ILT3 expression on monocytes and conventional DCs correlated with decreased OS. On the other hand, CD11c on monocytes was a predictor of better outcome, and PD-L1 on conventional DCs was associated with longer PFS.
Single-cell analysis of cells treated with the ATP synthase inhibitor oligomycin and clustering based on 21 immune markers grouped monocyte and DC subsets separately. Among conventional DCs, cDC1s have the highest proportion of cells with mitochondrial respiration, and most of the pre-DC, CD5+ cDC2, and DC3 populations use glycolytic metabolism. HLA-DR expression was not impacted by metabolic state, while ILT3 and PD-L1 expression on most subsets was differentially expressed on glycolytic and mitochondrial populations, respectively. The metabolic state of ‘intermediate’ monocyte populations (partially overlapping with classical and nonclassical monocytes) was the best predictor of the metabolic state of mDCs.
Overall this work provides a detailed metabolic characterization of circulating precursors and culture-derived DCs with important insights into differences between vaccine responding and non-responding patients and healthy donors. Ex vivo culture of monocytes derived from patients with cancer using current methods may not result in DC with ideal metabolic activity and immune stimulatory phenotypes. These data may thus inform work to improve DC-based vaccines.
Write-up by Maartje Wouters, image by Ute Burkhardt.
Meet the researcher
This week, first author Juraj Adamik answered our questions.

What was the most surprising finding of this study for you?
The most surprising finding was the profound difference in metabolic activity between patient-derived dendritic cells (DC) used for cancer vaccines and DCs from healthy donors. DC cultured the same way used very different fuels for their activity. One of the challenges was the integration of the different metabolic bulk and single cell platforms and measurements used for profiling the patient dendritic cells, and their analysis.
What is the outlook?
Metabolism plays a more crucial role in the effectiveness of DC-based immune cell therapies than previously appreciated. Therefore, the modulation of metabolic parameters or metabolites, such as lactate, is important. Metabolism is a biomarker for cell health, and may be a new addition to immune phenotyping.
What was the coolest thing you’ve learned (about) recently outside of work?
As a stamp collector, I was fascinated to discover recent news: the Inverted Jenny, an exceptionally rare stamp featuring an upside-down vintage airplane, sold for a staggering 2 million dollars.




