We know that when oxygen is lacking, T cells increase their glucose uptake and glycolysis, and when glucose is lacking, cells switch to oxidative phosphorylation (OXPHOS), which can utilize many substrates for fuel, but requires oxygen. But what happens to the T cells in the tumor microenvironment (TME), which is often hypoxic and hypoglycemic at the same time?
Zhang et al. utilized a transplantable mouse melanoma model in an attempt to answer this question. The mice were injected with a mixture of two vaccines: one vaccine that induced melanoma-associated antigen (MAA)-specific CD8+ T cells and another that induced irrelevant human papillomavirus protein E7-specific CD8+ T cells. The team found that, contrary to popular belief, CD8+ tumor-infiltrating T cells (TILs) became functionally exhausted regardless of antigen specificity, indicating that chronic antigen stimulation was not required for TIL exhaustion. Using adoptively transferred T cells in a different mouse model, the researchers showed that TIL exhaustion was not an effect unique to their vaccination model.
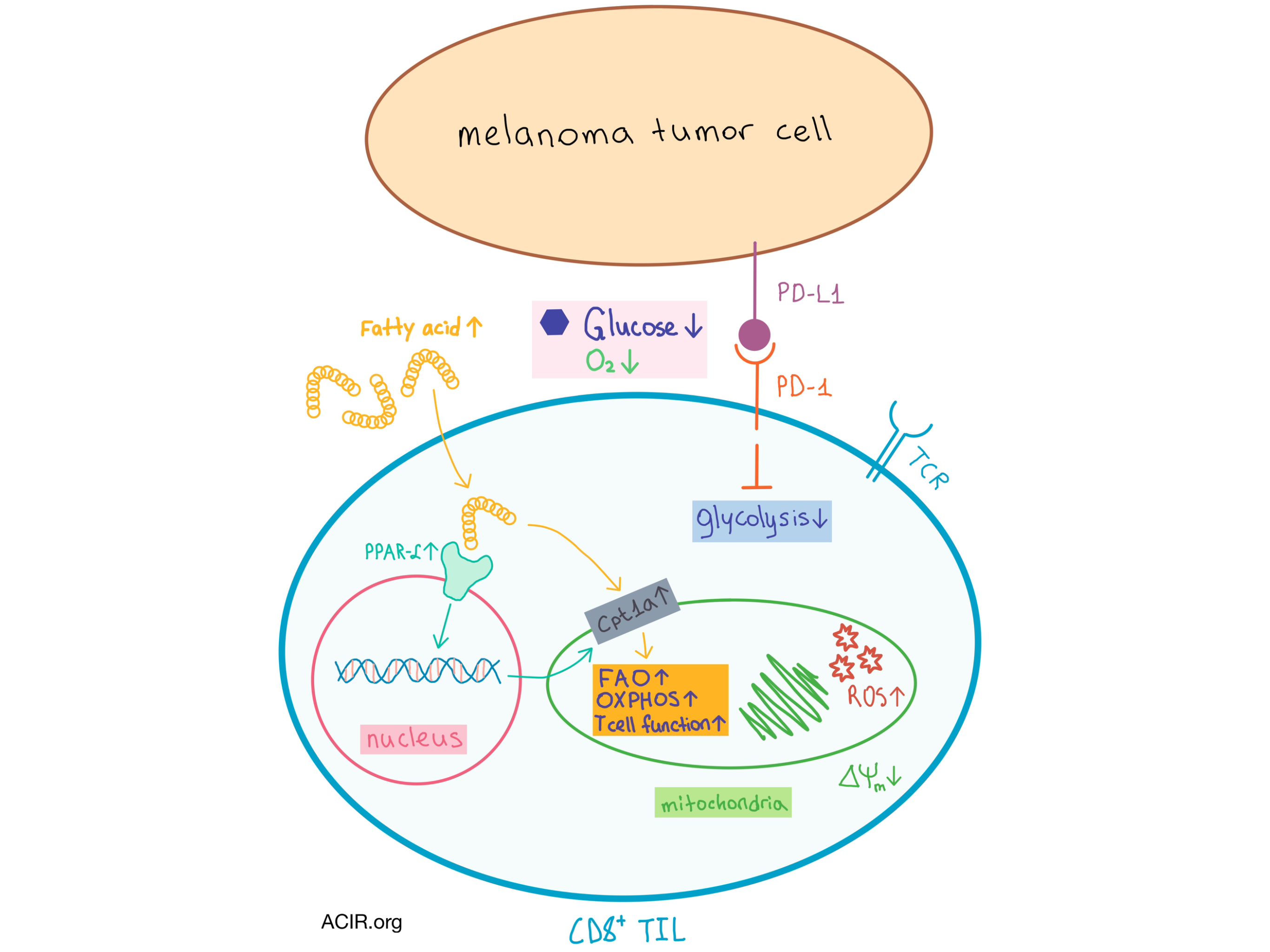
Building on a hypothesis that metabolic changes caused by the TME led to TIL exhaustion, which could affect both tumor-antigen specific and irrelevant T cells, the team searched for effects of hypoxia. In in vivo studies, MAA- and E7-specific CD8+ TILs were exposed to hypoxia during tumor progression, as indicated by the increased expression of hypoxia-inducible factor 1α (HIF-1α) – the main transcriptional regulator in response to hypoxic conditions. Hypoxia also led to an increase in the inhibitory immune checkpoint receptor LAG-3 (but not PD-1) and impaired TIL function, and these effects could be reversed in vitro and in vivo by shRNA knockdown of HIF-1α. The TILs also upregulated Glut1, the downstream target of HIF-1α that facilitates glucose uptake. However, upregulation of glycolysis in the hypoxic and hypoglycemic TME could be futile, so how would cells adapt to both limitations?
In vitro and in vivo studies showed that under simultaneously hypoxic and hypoglycemic conditions, TILs decreased glycolysis, increased OXPHOS, preserved effector function, and, importantly, increased expression of peroxisome proliferator-activated receptor α (PPAR-α), a key nuclear transcription factor that promotes fatty acid (FA) uptake and catabolism. Interestingly, these changes were not observed in splenic CD8+ T cells, confirming the effect of the TME on T cell metabolism.
The metabolic switch of TILs is facilitated by an enhanced uptake of FAs, which are found in abundance within the tumor interstitial fluid in melanoma mice during tumor progression, as well as the increased expression of the FA β-oxidation (FAO) rate-limiting enzyme Cpt1a, as observed in vaccine-induced CD8+ TILs from late-stage tumors. Importantly, increased levels of PPAR-α, Cpt1a, and FA uptake are observed in CD8+ TILs from melanoma patients, suggesting that the switch toward FA catabolism also occurs in human cancers.
In addition to metabolic changes, PD-1 expression in TILs in the TME also increased over time. Treatment of mice with anti-PD-1 delayed tumor progression but did not affect the metabolism or function of TILs. In a separate in vitro experiment, under simultaneously hypoxic and hypoglycemic conditions, the researchers treated CD8+ T cells with fenofibrate, a PPAR-α agonist, and observed increases in granzyme-B production, FA catabolism, and T cell function, and a concurrent increase in PD-1 expression. These results, which were confirmed in vivo (where fenofibrate significantly delayed tumor progression), suggest that PD-1 expression does not necessarily indicate impaired TIL function. In fact, PD-1 signaling inhibited certain T cell activating pathways, resulting in decreased glycolysis and increased lipolysis and FAO. Thus, PD-1 expression facilitates the metabolic switch of TILs, which then persist employing the FA catabolic pathway regardless of further PD-1 signaling.
When fenofibrate and anti-PD-1 treatments were combined in vivo, they acted synergistically to delay tumor progression and completely prevent tumor growth in 50% of vaccinated mice. These results were promising, but it is important to note that energy production via FA catabolism rather than glycolysis comes with a hefty price tag: FAO requires more oxygen to create an equivalent amount of energy, which leads to increased production of reactive oxygen species within the mitochondria, decreased mitochondrial membrane potential, and deformed cristae and membrane structures, likely resulting in a search for additional energy sources.
Overall, the results of this study suggest that metabolic reprogramming to FA catabolism of CD8+ T cells being prepared for adoptive cell transfer may benefit patients with hypoglycemic tumors, and that this metabolic switch can synergistically combine with PD-1 blockade to reduce tumor progression.
by Anna Scherer




