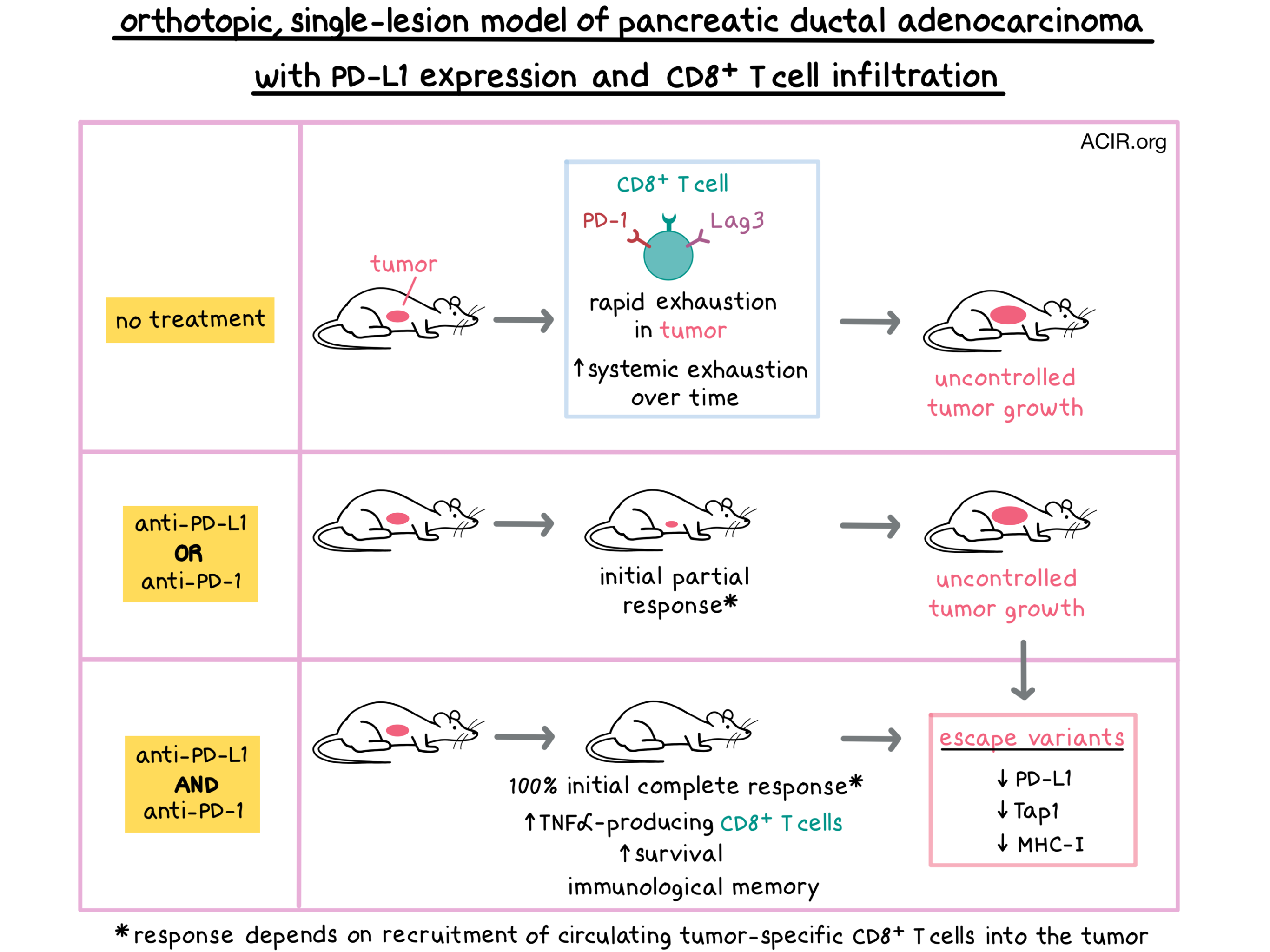
Aiming to elucidate the role that neoantigens play in the response of pancreatic ductal adenocarcinoma (PDA) to immunotherapy, particularly in a subset of patients with PDA that is infiltrated by T cells, Burrack et al. developed a new mouse model expressing a neoantigen to study the fate of tumor-specific CD8+ T cells during immune checkpoint blockade. The results were recently published in Cell Reports.
The researchers began by isolating primary tumor epithelial cells from the well-studied multi-lesional KPC mouse tumor model to develop a transplantable orthotopic model that establishes a single tumor. To enable imaging, tumor epithelial cells were transduced with a retrovirus expressing click beetle red (CB) linked to enhanced green fluorescent protein (eGFP). A CB-eGFP+ tumor clone (KPC2a) was identified that was surprisingly responsive to short-term PD-L1 blockade, with decreased tumor growth and prolonged survival compared with parental KPC tumors.
Examining the mechanism behind the response to immunotherapy, the researchers found that KPC2a tumors had significantly increased PD-L1 expression and intratumoral CD8+ T cell frequency compared with autochthonous KPC PDA tumors. Anti-PD-L1 treatment did not affect intratumoral CD8+ T cell frequency, but in the spleen it did increase the frequency (but not number) of CD8+ T cells in general and PD-1+LFA-1+ CD8+ T cells in particular (indicating enhanced T cell priming). Thus, this new PDA tumor model may be useful for modeling a subset of human PDAs that are infiltrated by CD8+ T cells and express PD-L1.
Hypothesizing that the CB protein generated a bona fide tumor-controlling neoantigen, Burrack et al. demonstrated that the response to anti-PD-L1 was dependent on CB expression and identified an H-2Db-restricted peptide, CB101-109, that was eliciting a CD8+ T cell response. Armed with this knowledge, the researchers created a CB101-109:H-2Db fluorescently labeled tetramer and used it to detect T cells with the corresponding TCR in B6 mice. Interestingly, tetramer staining showed that the precursor frequency of naive CD8+ T cells in B6 mice was about 10-fold lower than the precursor frequency for the strong OVA SIINFEKL antigen.
Tetramer staining showed that anti-PD-L1 treatment increased the frequency and number of CB-specific T cells in the blood and in the spleen, but not in the tumor. Furthermore, treating mice with FTY720 to sequester lymphocytes within lymph nodes prior to anti-PD-L1 demonstrated that recruitment of peripheral T cells into the tumor was required for an anti-PD-L1-mediated antitumor effect.
Next, the researchers examined the phenotype of the CB-specific T cells over time. At 22 days post tumor implantation, a higher proportion of tetramer-positive T cells showed a dysfunctional phenotype in the tumor compared with the spleen, as indicated by the increased expression of PD-1, Lag3, Tim3, and TIGIT. Over time, CB-specific T cells in the spleen also became progressively more dysfunctional, as indicated by an increase in the proportion of PD-1+ and Lag3+ cells. Anti-PD-L1 decreased the proportion of CB-specific T cells that co-expressed multiple inhibitory receptors and increased the proportion of IFNγ-producing T cells in the tumor and in the spleen. While the number of tetramer-positive T cells increased in the spleen over time, it decreased in the tumor, with or without anti-PD-L1 treatment. Tetramer-positive T cells in the blood of mice treated with anti-PD-L1 had a CD44hiCD62L- effector phenotype. The majority of circulating CB-specific T cells were KLRG1+, while their intratumoral counterparts were mostly KLRG1-.
Given the increasing dysfunction of T cells over time, Burrack et al. tested the effect of prolonged treatment with anti-PD-L1, anti-PD-1, or a combination of the two antibodies on tumor-bearing mice. Mice treated with either monotherapy initially responded to treatment with a decrease in tumor size, but the tumors eventually grew in most mice. Prolonged anti-PD-L1 treatment did not improve survival compared with short-term anti-PD-L1. In contrast, all mice treated with dual blockade had complete tumor regression by day 14. After cessation of therapy, tumors cyclically recurred and shrunk, indicating immunological memory. 50% of mice treated with dual blockade survived indefinitely, compared with 20% of mice in each monotherapy group.
Although circulating CB-specific T cells increased in number in all treated mice at 2 weeks post-treatment, they became undetectable in monotherapy-treated long-term survivors after tumor clearance, suggesting that antigen presentation was required for persistence. Interestingly, in long-term survivors treated with dual therapy, CB-specific T cells persisted and eventually re-expressed the central memory marker CD62L, suggesting an early imprinting of checkpoint therapy on the phenotype of the re-invigorated cells. In the tumor, dual blockade increased the proportion of KLRG1+Lag3- and TNFα-producing CB-specific T cells, and decreased the proportion of PD-1+ CB-specific T cells.
Finally, the researchers examined the mechanisms of immune evasion in tumors that escaped after anti-PD-L1 therapy. Although the escape variants had detectable levels of CD8+ T cells, these tumors had low expression of PD-L1 and demonstrated defects related to antigen processing and presentation, including reduced MHC I levels due to reduced induction of Transporter associated with Antigen Processing 1 gene (Tap1) expression following co-culture with IFNγ. Some tumor escape variants also had defects in the expression of immunoproteasome units Lmp2 or Lmp7. These results suggest that defects in Tap1 and MHC-I expression after IFNγ signaling may lead to immunotherapy resistance in PDA due to lack of antigen presentation.
Overall, Burrack et al. used their new model to show that tumor-specific CD8+ T cells became rapidly exhausted within PDA tumors and, over time, in the spleen as well. Treatment with anti-PD-L1 or anti-PD-1 induced an antitumor response that was dependent on recruitment of tumor-specific circulating T cells into the tumor, but ultimately neither monotherapy could control tumor growth. However, combination of anti-PD-L1 and anti-PD-1 reduced tumor growth, improved survival, and induced immunological memory, which conferred long-term tumor control, supporting clinical investigation of this approach.
by Anna Scherer
Meet the researcher
This week, lead author Ingunn Stromnes answered our questions.
What prompted you to tackle this research question?
Our goal is to design and tailor T cell therapies to be effective for eradicating pancreatic cancer, a deadly disease with few effective therapies. While current immunotherapies are effective in some cancers, pancreatic cancer has evaded immunotherapy. Thus, it is compelling for us to develop models in which we can study the rare T cells that matter, e.g., the T cells that specifically recognize pancreatic tumor cells to investigate the underlying biology. If we can understand how T cells can eliminate pancreatic tumors, as well as how pancreatic cancer evades the immune system, we will be well equipped to tackle this disease by harnessing the immune system.
What was the most surprising finding of this study for you?
Perhaps one of the most surprising findings is how well T cells can infiltrate pancreatic tumors. The dogma is that pancreatic cancer prevents T cell infiltration and that is the reason why this disease resists immunotherapy. That may indeed be part of the problem, yet is clearly not the entire story. We find that T cells that are tumor antigen-specific readily and preferentially localize inside large bulky pancreatic tumors in animals. This is entirely consistent with our prior study of an engineered T cell therapy. We demonstrate that once T cells infiltrate PDA, they are rapidly rendered dysfunctional. An additional surprising finding is that there is synergy by combining PD-1+PD-L1 blockade. More and more studies suggest that these antibodies may not mechanistically be acting in the same way, despite targeting a similar pathway. We are also quite intrigued with identifying a potential putative subpopulation of tumor antigen-specific T cells that may have qualities that render them superior at eliminating pancreatic cancer, and this is a major area of our lab's current investigation.
A potentially less surprising finding, yet equally important, is the fact that pancreatic tumor cells that evade immunotherapy lose expression of one particular gene critical for antigen processing and presentation. This result will hopefully lead us into identifying ways to overcome this resistance mechanism.
What was the coolest thing you’ve learned (about) recently outside of work?
This is probably the most challenging question because so much of my life is focused on the science in the lab. I am a runner and I thought this was a cool article on a young woman who lost both parents to cancer in a matter of months and chose to run 4,000 miles across the country with others to help heal. It shows the strength of the human spirit and how working together as a team for a common goal can help overcome at least some of the grief faced following the loss of a loved one. https://www.runnersworld.com/runners-stories/a28722895/caroline-watson-runs-in-honor-of-parents-cancer-deaths/




