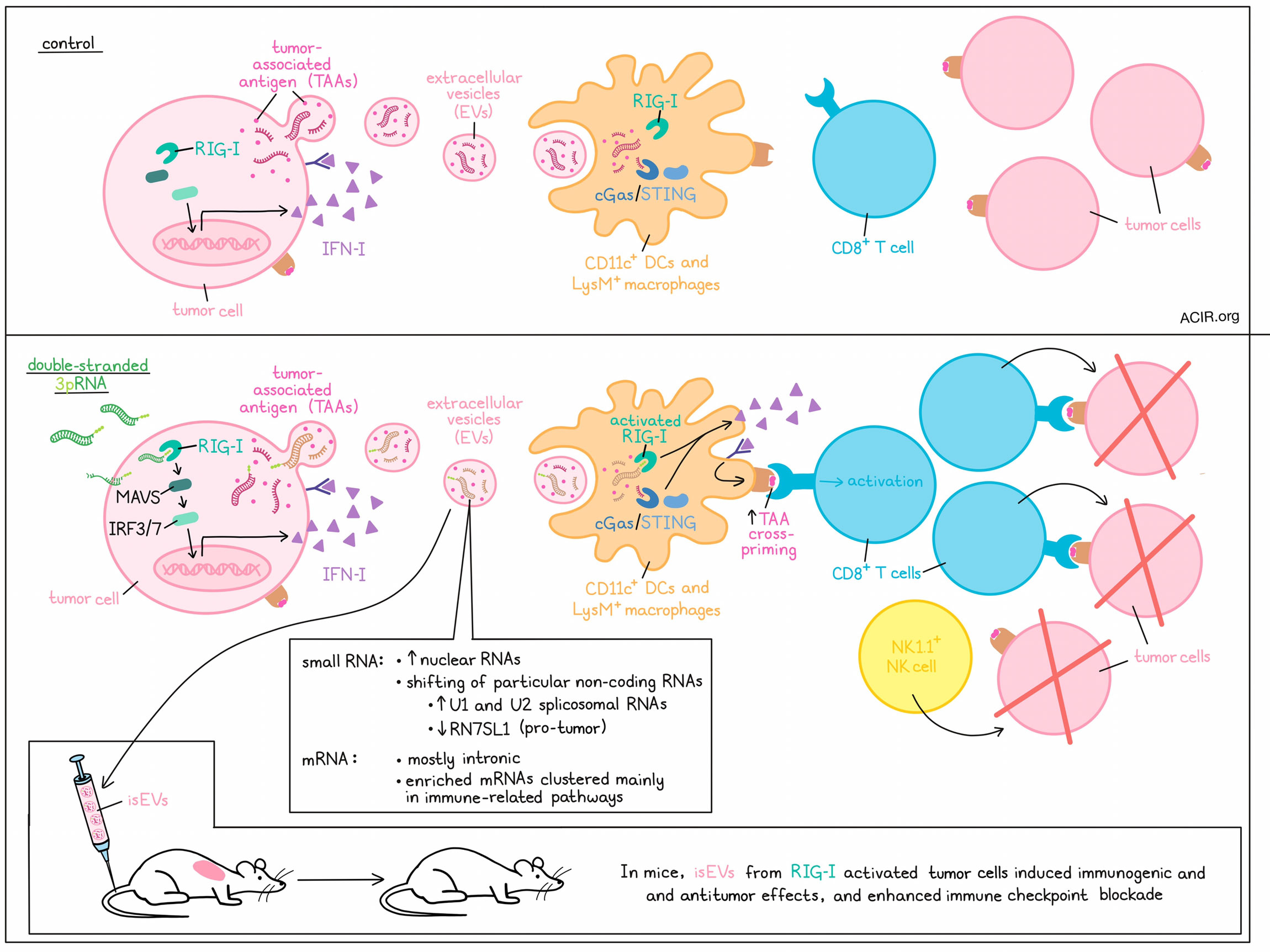
As a form of communication/signaling, cells frequently release extracellular vesicles (EVs). While EVs released by cancer cells have previously been associated with immune evasion and cancer progression, they can also potentially carry tumor antigens and stimulate immune responses. Investigating the mechanisms that govern cancer EV biogenesis, cargo, and functions, Heidegger and Stritzke et al. found that engagement of the cytosolic RNA receptor RIG-I could alter the cargo of EVs, inducing the generation of EVs capable of activating innate immunity and subsequent antitumor T cell responses. The results were recently published in Cell Reports Medicine.
Based on prior evidence that RIG-I activity in tumors enhances the immunogenicity of EVs, Heidegger and Stritzke et al. used B16.OVA melanoma cells transfected with double-stranded 3pRNA – a well established RIG-I ligand characterized by a terminal triphosphate – and enriched EVs, which contained multiple proteins typically found in exosomes, from the supernatant. While neither activation by 3pRNA nor knockout of RIG-I appeared to impact the size, protein load, or number of EVs released, RIG-I-EVs induced strong maturation and IFN-I production in DCs in a dose-dependent manner. For comparison, EVs from melanoma cells activated for cGAS/STING induced only weak maturation and IFN-I production in DCs, and EVs from control melanoma cells did not stimulate DCs.
Analyzing the molecular mechanisms at play, the researchers found that the enhanced immunostimulatory capacity of RIG-I EVs was dependent on both increased IRF3/7 transcription factor activity and increased IFN-I production downstream of RIG-I. This function did not appear to be related to IFN-I encapsulated in EVs, nor due to induction of programmed cell death. Similar results were observed in several other cancer lines and in non-transformed fibroblasts.
Looking more closely at the content of EVs, Heidegger and Stritzke et al. found that they carried both exogenous and endogenous tumor-associated antigens, including OVA and gp100, but that this was not influenced by RIG-I. Still, immunization of mice with immunostimulatory RIG-I-EVs (isEVs) from melanoma cells, even without OVA, induced strong immunogenic effects (including CD8+ and CD4+ T cell activation and increased mature DCs in the TME), and antitumor activity (inducing tumor growth delay, prolonged survival, and protection from rechallenge in cured mice). Antitumor efficacy was found to be dependent on both CD8+ T cells and NK1.1+ NK cells. Further, RIG-I-EVs enhanced sensitivity to immune checkpoint blockade (anti-PD-1/-CTLA-4) in settings of resistance in several models, suggesting that isEVs could act as personalized vaccines to enhance immunotherapy. To test this potential function, the researchers generated EVs from short-term cultures of tumor tissue resected from mice. Like the isEVs derived directly from homogenous tumor cell lines, EVs from heterogeneous tumors showed immunostimulatory functions.
Upon interacting with DCs, tumor EVs were found to be taken up and delivered to the cytosol via endocytosis and macropinocytosis, though only macropinocytosis of EVs resulted in IFN-I induction. In past research, macropinocytosis of EVs has been associated with cytosolic delivery of nucleic acid cargo, which could potentially be detected by RIG-I/MAVs and/or cGAS/STING in innate immune cells, inducing potent stimulation. Using models in which the RIG-I or cGAS/STING pathways were knocked out in host immune cells, the researchers showed that both were essential for strong IFN-I induction and subsequent T cell activation in response to isEVs, though RIG-I contributed more. Further, IFN-I induction from both CD11c+ DCs or LysM+ macrophages was essential for T cell activation. These data suggest that the immunogenicity of RIG-EVs likely stems from cargo that activates RIG-I/MAVS (and to a lesser extent cGAS/STING) in innate immune cells.
Using fluorescence to label the bulk RNA cargo of tumor EV samples, the researchers observed concentration-dependent transfer of RNA from EVs to innate immune cells. When they extracted nucleic acids from tumor-cell-derived EV samples and generated liposome-bound EV RNA, only DCs transfected with RNA from RIG-I-activated cells produced IFN-I. When the terminal triphosphate of isEV RNAs was cleaved off, the immunostimulatory capacity was reduced, but not abrogated, suggesting that immunostimulatory isEV-RNA is heterogeneous and is composed of both RIG-I-activating 3pRNAs and other non-3p-containing, RIG-I-targeting stimulatory RNAs. Only a very small portion of these 3pRNAs were from those initially used to stimulate the tumor samples.
Analyzing small RNAs in EVs, the researchers found that RIG-I signaling in tumor cells shaped global composition of the RNA, enriching small nuclear RNAs and shifting the abundance of particular non-coding RNAs, including small nucleolar RNAs. U1 and U2 spliceosomal RNAs were particularly enriched, while the pro-tumoral RIG-I-activating endogenous ncRNA RN7SL1 was markedly reduced in RIG-I-EVs. Most mRNA that could be identified was linked to intronic vs exonic regions, and significantly enriched mRNAs mainly clustered in immune-related pathways.
Finally, the researchers investigated the translatability of their findings by looking at EVs enriched from supernatants of the human melanoma cell line D04mel. Experiments using this cell line showed similar results to their murine counterparts, with 3pRNA-mediated RIG-I signaling enhancing the immunogenicity of EVs and triggering innate immune activation and cross-priming of tumor antigen-specific CD8+ T cells. From past clinical data, the researchers identified a tumor EV pathway gene set that was associated with reduced OS in patients with malignant melanoma. When patients with high expression of this unfavorable gene set were stratified by median expression of DDX58 (encoding RIG-I), higher DDX58 expression was associated with significantly prolonged survival, though neither the tumor EV pathway gene set expression nor low DDX58 expression were independent risk factors for death. Another more broadly defined EV gene signature, deemed the common EV pathway gene set, showed a strong correlation with DDX58 expression, but not with overall survival.
Overall, these results show that 3pRNA-mediated stimulation of RIG-I in tumors induces IFN-I expression and alters the RNA cargo of EVs, enhancing their immunostimulatory potential. These isEVs are then capable of activating innate immune cells through RIG-I and cGAS/STING, inducing IFN-I production and maturation, and subsequently, activation of tumor-specific CD8+ T cells that mediate antitumor immunity. RIG-I stimulation and/or isEVs themselves could potentially be used to enhance antitumor immune responses in support of immune checkpoint blockade.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, co-first author Simon Heidegger and lead author Hendrik Poeck answered our questions.

What was the most surprising finding of this study for you?
The fact that we could completely reprogram the immunogenic function of tumor-derived extracellular vesicles (EV) by activating a specific innate immune pathway within tumor cells. Therefore, we simply needed to add a synthetic RNA ligand to cell cultures.
In the process of this project, it was fascinating for us to learn how limited the current understanding of EV biology is in general. On the flip side of the coin, there are so many amazing things still left to be uncovered in the EV field and associated therapeutics.
What is the outlook?
Before we can think of translation to clinical use, we will need to further investigate the mechanisms that modulate secretion of these immunostimulatory tumor-derived (isEVs). We will also explore isEVs in combination with other immunotherapies (such as CAR T cells) in advanced preclinical cancer models, including human organoid-based systems and xenografts. The field in general will need to overcome current limitations and lack of standardization in large-scale, good manufacturing practice (GMP)-grade purification of EVs for use in patients.
What was the coolest thing you’ve learned (about) recently outside of work?
HP: That California is a beautiful state with breathtaking shores and national parks.
SH: I recently got to know the story of Ernest Shackleton and his failed expedition into Antarctica, their ship wreckage, and the fact that he and his crew were stuck in pack ice for more than a year. It is fascinating what obstacles humans can overcome, and what trials they can endure. I can only recommend anyone to read up on this fascinating and true story.




