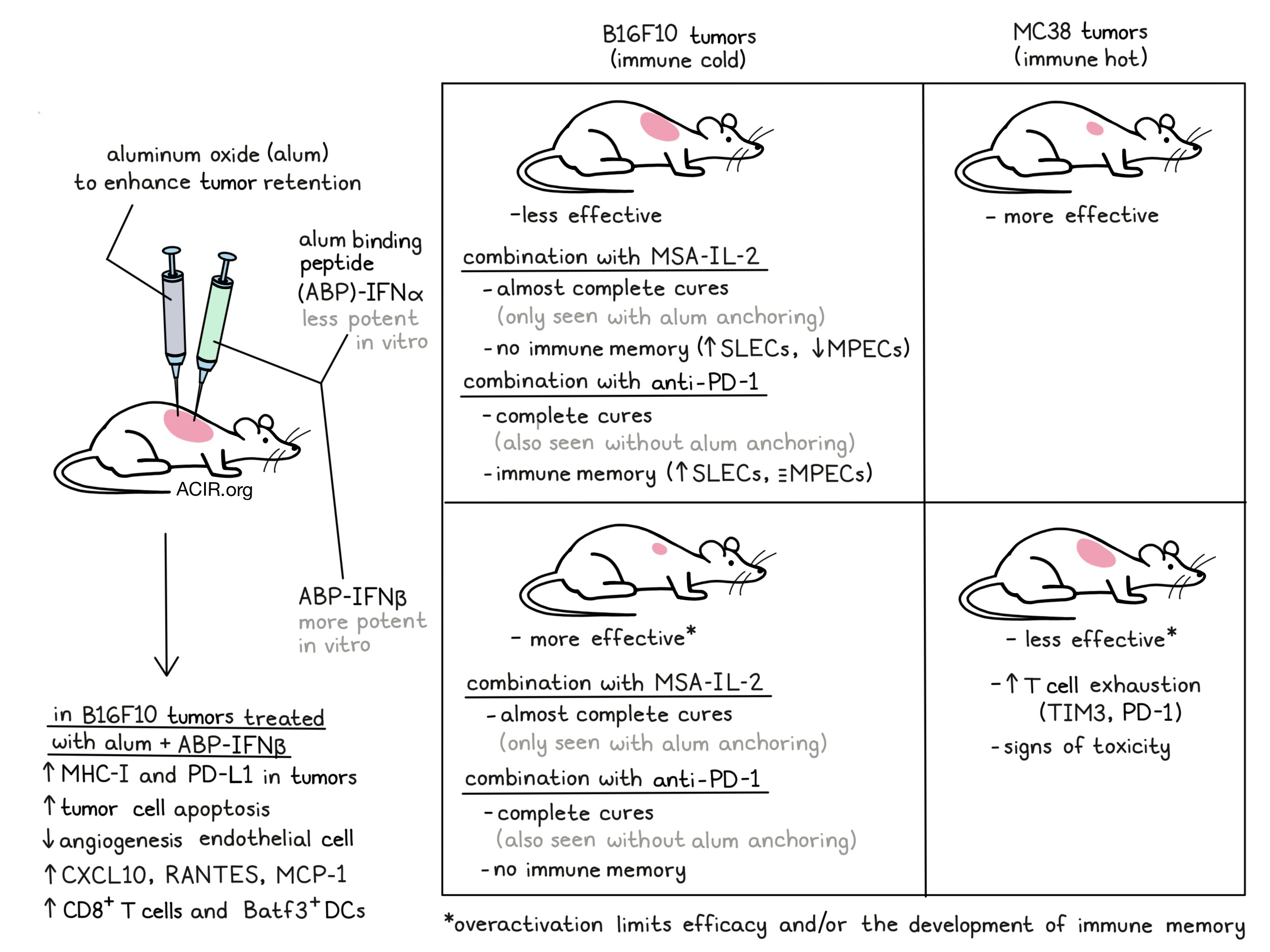
While intratumoral therapy with type I interferons (IFNs) may have favorable antitumor effects, the treatment is limited by rapid tumor clearance and off-target systemic toxicity. To circumvent these issues, Lutz et al. developed a strategy in which engineered IFNs are co-injected with aluminum-hydroxide (alum) particles, which anchor them in the tumor microenvironment. Data assessing this strategy in mouse models was recently published in PNAS.
The researchers developed five type I IFNs for this study; murine IFNα subtype A (IFNα), murine IFNβ, IFNα fused to mouse serum albumin (MSA) to increase half-life, and aluminum oxide (alum)-binding peptide (ABP)-IFNα or -IFNβ. ABP cytokines were mixed and co-injected with alum to retain these IFNs at the injection site. The IFNβ constructs were shown to be more potent than the IFNα constructs in vitro. The pharmacokinetics of these constructs was assessed in the B16F10 melanoma model. When co-injected with alum, ABP-IFNα and ABP-IFNβ retained in the tumor for five days after intratumoral injection, while IFNα, IFNβ, and MSA-IFNα leaked out of the tumor on day one.
The therapeutic efficacy of the IFNs as intratumoral monotherapies was assessed in the MC38 colon carcinoma and the B16F10 models. While IFNα and IFNβ only extended median survival for a couple of days, intratumoral retention improved outcomes, with the alum + ABP-IFN versions being more efficacious than their non-anchored counterparts. Alum + ABP-IFNβ was most efficacious in the immunological cold B16F10 model, while in the MC38 model, the less potent alum + ABP-IFNα worked better. The latter tumor type has higher basal type I IFN signaling and more immune infiltration, suggesting there may be a threshold for effective type I signaling, with overactivation limiting efficacy. Confirming this, in MC38 tumors treated with alum + ABP-IFNβ, CD8+ T cells expressed higher levels of exhaustion markers TIM3 and PD-L1. While the efficacy of MSA-IFNα and alum + ABP-IFNα was similar, the former induced body weight loss, suggesting toxicity.
Using the immune cold B16F10 model and the potent alum + ABP-IFNβ, the researchers assessed how the intratumoral retention resulted in higher efficacy. This was assessed in WT mice and Ifnar1-/- mice, which have cells deficient in type I IFN sensing, so only the tumor cells could respond to the treatment. Treatment with alum + ABP-IFNβ resulted in higher expression of MHC-I and PD-L1 on the tumor cells in both mice types, though less pronounced in the Ifnar1-/- mice. The WT mice’s tumor cells were more apoptotic after treatment, an effect not seen in the Ifnar-/- mice, suggesting indirect IFN effects.
Given that type I IFNs are antiangiogenic, the researchers assessed the role of endothelial cells by generating bone marrow chimeras. WT and Ifnar1-/- mice were lethally irradiated and reconstituted with bone marrow from WT donors, so that in the Ifnar1-/- mice, only the non-immune cells were deficient in type I IFN signaling. After engraftment, the mice were inoculated with B16F10 cells and treated with alum + ABP-IFNβ. This strategy was more efficacious in the WT mice than in the Ifnar1-/- mice, suggesting an important role for interferon signaling in non-hematopoietic cells.
To further assess the immune response to treatment, B16F10 tumors were treated and harvested after four days for analysis. Compared to IFNβ alone, the alum-anchored treatment resulted in higher expression of CXCL10, RANTES, and MCP-1. Genetic knockouts or antibody-mediated depletions were performed to assess the role of specific immune populations, revealing that NK cells and neutrophils played no significant role in the efficacy of alum + ABP-IFNβ. However, lack of Batf3+ dendritic cells or CD8+ T cells decreased efficacy. The role of DCs and CD8+ T cells was further studied by assessing the tumor-draining lymph nodes of treated mice, where the frequency and total count of Ly6C-MHC-II+CD24+CD86+ DCs increased; two days later the proportion of Ki67+TIM3+TCF1+CD8+ T cells in the tumor was increased.
Next, the combination of extended half-life IL-2 (MSA-IL2) with IFNs was assessed in the B16F10 model. MSA-IL-2 alone increased survival by only two days, while the combination with alum + ABP-IFNα or alum + ABP-IFNβ resulted in almost complete cures, which was not the case when combined with the non-anchored IFNs. The researchers also studied the combination treatment with anti-PD-1 in this model. While anti-PD-1 alone did not cure mice, combining it with IFNα, alum + ABP-IFNα, IFNβ, or alum + ABP-IFNβ was curative in most cases, without any overt toxicities.
Surviving mice were rechallenged with B16F10 cells in the opposite flank to determine immune memory formation. Only previous treatment with anti-PD-1 combined with either IFNα, IFNβ, or alum + ABP-IFNα induced resistance to this rechallenge in the majority of mice. There was a clear contrast between MSA-IL-2 with alum + ABP-IFNα treatment and anti-PD-1 with alum + ABP-IFNα treatment. While both therapies were efficacious as primary treatments, only the anti-PD-1 with alum + ABP-IFNα treatment provided protection from rechallenge. To investigate the mechanism behind these effects, the researchers evaluated the spleens of treated mice. This revealed that the MSA-IL-2 with alum + ABP-IFNα combination resulted in an increase in the percentage of short-lived effector cells (SLECs) within the CD8 compartment, while it decreased the percentage of memory precursor effector cells (MPECs). In contrast, the anti-PD-1 combination also resulted in an increase in the percentage of SLECs, but the percentage of MPECs was largely maintained. Other differences between the groups included enlarged spleens and increased frequencies of CD8+ T cells expressing CD25, Ki67, and TIM3 in the MSA-IL-2 combination group. This data suggests that MSA-IL-2 and alum + ABP-IFNα combination treatment reduced MPECs and induced exhaustion markers on CD8+ T cells, which may explain the lack of protection against tumor rechallenge.
This study showed that treatment with alum-anchored type I IFNs may improve efficacy, limit toxicity, and can be combined with other immunotherapeutics. However, this study also revealed the importance of considering the tumor environment to determine the best therapeutic strategy for interferons, as their overstimulation may backfire.
Write-up by Maartje Wouters, image by Ute Burkhardt
Meet the Researcher
This week, first author Emi Lutz and lead author Dane Wittrup answered our questions.

What was the most surprising finding of this study for you?
Combining intratumorally anchored interferon alpha with either systemic IL-2 or anti-PD-1 both led to cures of most established B16F10 tumors, yet only with checkpoint blockade was protective immune memory formed. This difference correlated with skewing of the T cell response towards MPECs over SLECs and could be critical for localized therapies such as ours to have impact on disseminated metastatic disease.
What is the outlook?
The alum-anchoring technology has been licensed to a startup company (Ankyra Therapeutics), that is planning to initiate a Phase I clinical trial in 2023. It will be tremendously exciting to see how human tumors respond to anchored cytokines.
What was the coolest thing you’ve learned (about) recently outside of work?
EL: The coolest thing I've learned about recently outside of work is the nature in Northern Sweden. On a backpacking trip, I got to see many reindeer and learned that their eyes change color based on the season to adapt to the changes in light. In the summer, there is 24 hours of sunlight, while in the winter, it is mostly dark.
DW: My son and I visited Walla Walla, Washington, and learned about unusual wines made from Syrah grapes grown in The Rocks AVA, which somehow imparts a strong reductive note that is charitably called funky. The wines have names like “Bionic Frog” and “The Funkadelic.” We loved them – but the rest of our family politely declined to partake.




