Therapeutic cancer vaccines consistently show great promise, but often fall short of expectations. Past studies have successfully used dendritic cells (DCs) to carry antigens to tumors, but DCs can be difficult to obtain and manipulate in large numbers. T cells, on the other hand, are readily available, easy to modify and expand (due to the extensive efforts on CAR T cells), and are known to induce endogenous T cell responses when carrying foreign antigens. Recently, Vaetch et al. tested the use of T cells as a vaccine vector; their results were published in The Journal of Clinical Investigation.
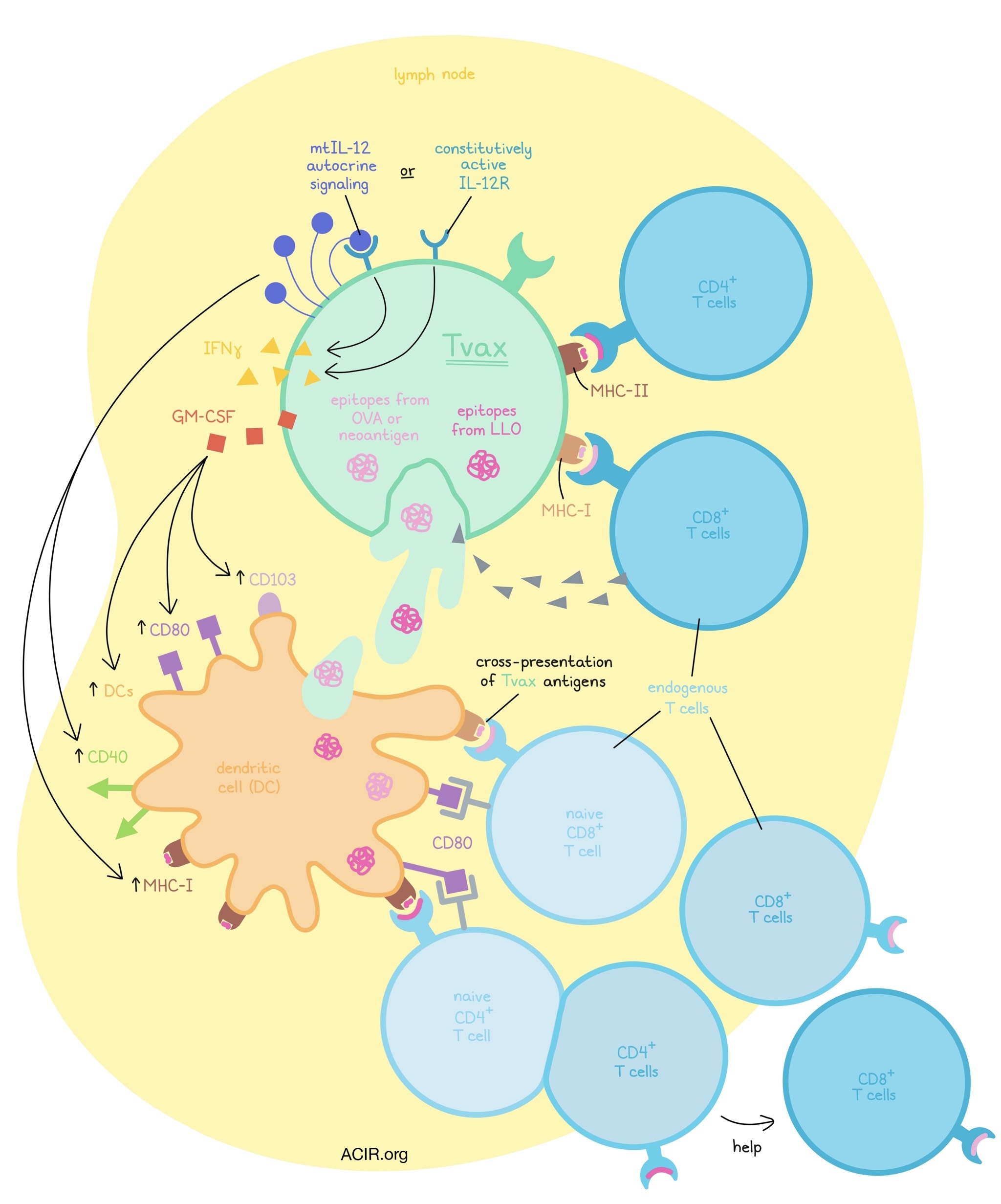
To develop their T cell vaccine (Tvax), Veatch et al. began by transducing murine T cells with a a truncated murine CD19 surface marker fused to an OVA epitope that presents on MHC-I, and the Listeria-derived LLO epitope, which presents on MHC-II. When delivered systemically into mice, a single dose of Tvax cells induced strong CD8+ T cell responses to OVA, and weaker CD4+ T cell responses to LLO. The CD4+ T cell response was shown to aid in the priming of the strong response to OVA. A fraction of the induced CD8+ T cells were also found to differentiate into a central memory phenotype, which responded strongly to a boost with a second Tvax dose.
Evaluating trafficking, the researchers found Tvax cells in the T cell zones of lymph nodes and in the white pulp of spleens. Tvax cells expressing OVA, but not control Tvax cells, were cleared within 14 days, consistent with immune-mediated rejection of cells carrying foreign antigens. Within the lymph nodes, CD11c+ DCs took up components of Tvax cells; DCs recovered from lymph nodes were able to cross-present antigen ex vivo, suggesting that this was primarily responsible for the induction of endogenous T cell responses. Tvax cells were also able to present antigens directly to naive T cells, and while this mechanism was not essential for strong early responses, it did contribute to the durability of responses.
Knowing that successful T cell priming requires more than just a strong peptide, Veatch et al. augmented Tvax cells with a variety of costimulatory and cytokine signaling molecules that could support DCs (GM-CSF, IFNβ, FLT3L, CD40L) and T cells (modified IL-2, CD137L, CD80, and membrane-tethered IL-12). The researchers found that Tvax modified with a combination of GM-CSF and mtIL-12 most potently enhanced endogenous T cell responses to Tvax, inducing a 25-fold increase over Tvax containing antigen alone.
The greater-magnitude responses induced with the inclusion of GM-CSF and mtIL-12 were associated with a higher frequency of effector cells, enhanced CD4+ T cell responses, increased polyfunctionality in CD8+ and CD4+ T cells, a higher absolute number of memory cells, and longer-lasting responses. Memory cells also responded strongly to a boost with Tvax, though interestingly, the inclusion of GM-CSF and mtIL-12 in the second dose of Tvax did not contribute any additional effect. Tvax with GM-CSF and mtIL-12 ultimately outperformed a peptide-pulsed bone marrow-derived DC vaccine at similar cell numbers.
Having shown that Tvax was capable of inducing potent responses to the foreign antigen OVA, the researcher next investigated whether it could induce similar responses to neoantigens. Tvax modified to express Alg8 and Lama4 epitopes (including LLO) induced robust endogenous T cell responses to these epitopes, which were enhanced by the inclusion of GM-CSF and mtIL-12, and by a boost with Tvax. Next, Tvax was used to target 6 neoantigens from the MC38 colon carcinoma model, which were predicted and observed to bind to MHC-I. While previous studies using standard peptide vaccine had only shown 3 of the 6 peptides to be immunogenic, Tvax induced responses to all 6 predicted neoantigens. However, when the researchers attempted to use Tvax to break tolerance to known self-antigens, they were unsuccessful.
Investigating how IL-12 enhances Tvax, the researchers found that IL-12 did not affect endogenous T cells, but instead acted on Tvax cells through autocrine signaling. This induced IFNγ production, which contributed to the enhanced priming of endogenous T cells, but was not wholly responsible. When mtIL-12 was replaced with a constitutively activated IL-12 receptor, the effect was similar, albeit slightly reduced, suggesting that this cell-intrinsic strategy could be substituted to limit potential toxicity associated with systemic IL-12.
The inclusion of mtIL-12 increased some activation markers on DCs, including CD40 and MHC-I, while the inclusion GM-CSF induced expression of nonoverlapping activation markers, including CD80. GM-CSF also increased expression of CD103 on cells that took up Tvax, consistent with recruitment of DCs to the spleen, and increased total DCs. Overall, IL-12 and GM-CSF appeared to work through mostly non-redundant mechanisms.
Testing the potential efficacy of Tvax in a tumor model, Veatch et al. used a transplantable B16 melanoma model expressing OVA. Tvax delivering OVA with GM-CSF and mtIL-12 induced strong T cell responses and delayed tumor outgrowth. In a B16-OVA lung metastasis model, the same treatment prolonged survival. In a neoantigen model, in which both the tumor and Tvax were carrying the Alg8 and Lama4 epitopes, Tvax again induced responses, though they were not as strong as those induced in the OVA models. The addition of anti-PD-1 alone to this regimen did not have an effect, but the addition of anti-PD-1, IL-2, and an anti-TRP1 antibody strongly improved antitumor efficacy.
To enhance the potential for practical clinical translation of Tvax, the researchers made Tvax cells from allogeneic donor mice. Compared to the syngeneic vaccine, the immunogenicity of HLA-matched allogeneic Tvax was slightly reduced, and the immunogenicity of HLA-mismatched Tvax was further reduced. Still, the potential for an off-the-shelf product remains an appealing prospect, as the responses induced by HLA-matched allogeneic Tvax were still strong. To test whether Tvax could be clinically relevant in human cells, the researchers engineered human T cells to express viral or human neoepitopes. After expansion, human Tvax cells induced an IFNγ response from endogenous CD8+ and CD4+ T cells that were reactive to the specific vaccine epitopes.
Together, these results by Veatch et al. show that T cells engineered to carry antigens and adjuvants can effectively be used as a vaccine vector in mice, delivering their payloads to lymphoid tissues, where DCs cross-present antigens to induce endogenous T cell responses strong enough to control tumor growth. This strategy could be further enhanced in combination with other therapies, and warrants further investigation.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Joshua Veatch answered our questions.
What prompted you to tackle this research question?
Immune checkpoint inhibitors that target negative regulatory pathways on T cells have clinical efficacy in a broad range of human cancer, but most patients either don’t benefit or respond only transiently to these treatments. In theory, being able to increase the numbers or function of T cells specific for cancer antigens could increase the efficacy of these treatments, but existing vaccine platforms have mostly been ineffective at inducing high-level T cell responses to cancer antigens in patients.
It has been noticed that when an immunogenic antigen is genetically inserted into a patient’s own T cells, a powerful T cell response is induced against that antigen, even in patients who are severely immunocompromised. In most T cell trials, this is undesirable, as it leads to immune rejection of the T cells that are intended to serve a therapeutic purpose, but we sought to take advantage of this phenomenon to create a new vaccine platform using T cells modified with cancer antigens to induce and augment T cell responses targeting those cancer antigens, which we call Tvax.
In a mouse model, we showed that Tvax works by delivering antigens to lymphoid organs throughout the body where they transfer antigens to host dendritic cells that initiate the immune response. We could stimulate even more potent T cell responses to the antigen by combining the antigen with other inflammatory signals in the same Tvax cells, eventually having a version of the Tvax that contained antigens and the inflammatory molecules IL-12 and GM-CSF. This Tvax showed therapeutic efficacy in some mouse cancer models, and showed an ability to induce T cell responses to a broad number of cancer antigens in mice. We also showed that we could make similar Tvax cells using human T cells.
What was the most surprising finding of this study for you?
One of the pathways that stimulated the activity of our vaccine the most was the cytokine IL-12. Unfortunately, IL-12 has also caused a lot of toxicity in human trials and we wanted to identify a way to be able to replace the effect of IL-12 on our vaccine without using IL-12 itself. We thought this would be difficult because we presumed that IL-12 in our vaccine was acting on CD4+ and CD8+ T cells in the host during the priming of new immune responses. We used either vaccine cells or recipient animals that lacked the IL-12 receptor to see where IL-12 signaling was needed for the vaccine, and found to our surprise, that the IL-12 receptor was only needed on the vaccine cells. This meant the important IL-12 signal for the vaccine was on the vaccine cells themselves, and that we could replace IL-12 in our vaccine with an IL-12 receptor engineered to always be on. The activated IL-12 receptor is what we are carrying forward to our human clinical trials to avoid potential off-target toxic effects of IL-12.
What was the coolest thing you’ve learned (about) recently outside of work?
With the pandemic, it has been challenging to find fun activities with my children outside, so we started flying remote-controlled airplanes. These are a lot of fun, but can get tossed around in the wind and can get stuck high in trees. My usual strategy is to throw a shoe up into the tree to try and knock the plane down but this runs the risk of also getting your shoe stuck in the tree, and potentially also your other shoe. Luckily, we were eventually able to knock them all down, and provide substantial amusement for the children in the process. But in all seriousness, one bonus of the pandemic is the motivation to find outdoor activities for the family




