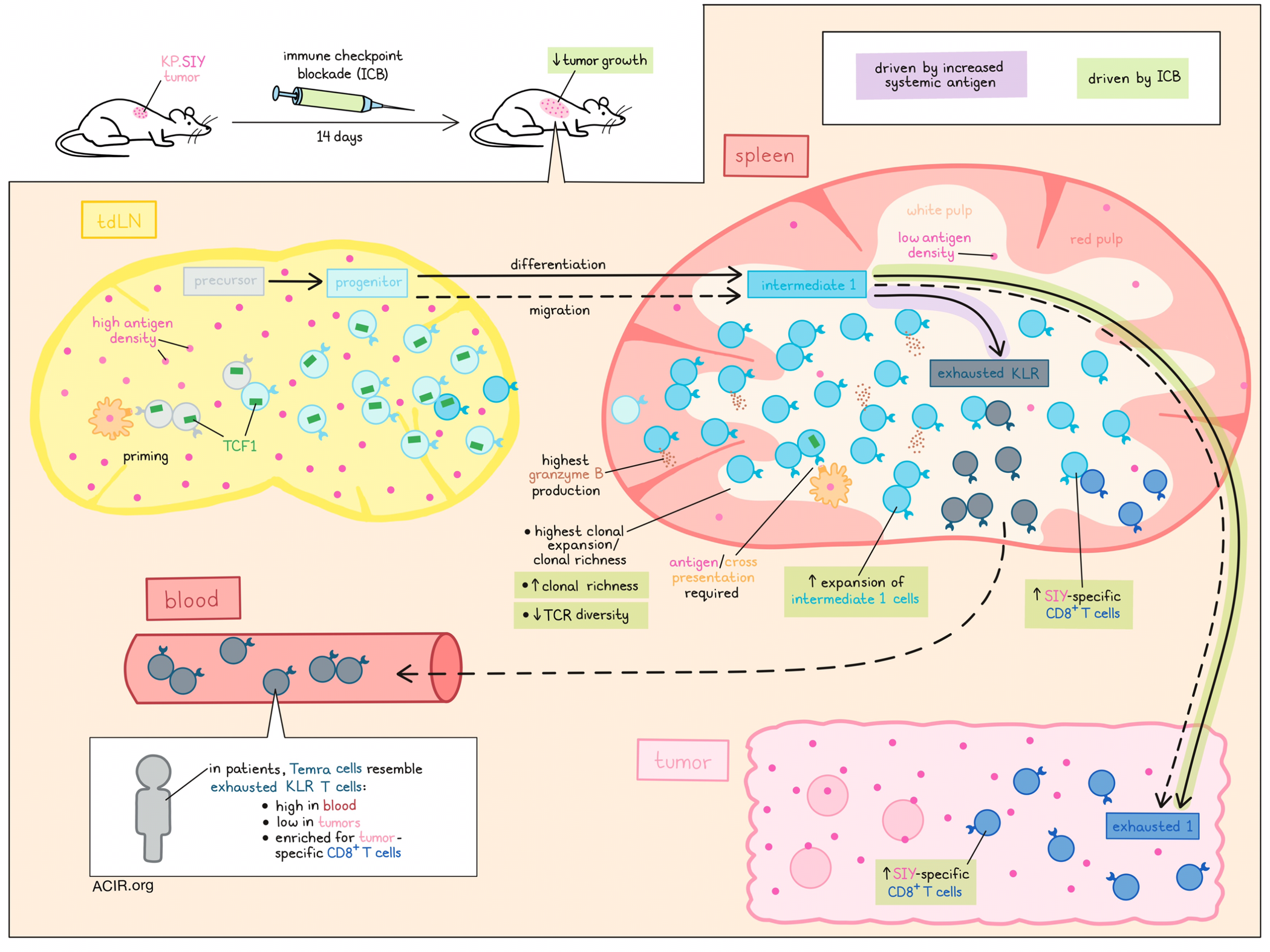
Immune checkpoint blockade unleashes powerful antitumor T cell responses, but exactly which T cell states are involved and from which anatomic locations they emerge are not fully understood. Investigating these topics, Morgan, Horton, Bhandarkar, et al. profiled T cells across tumors, lymph nodes, and spleens in tumor-bearing mice, and identified an intermediate-exhausted T cell population in the white pulp of spleens that gave rise to tumor-infiltrating exhausted T cells, dependent on optimal antigen exposure. Their results were recently published in Science Immunology.
To begin, mice were inoculated with KP lung tumors expressing the SIY antigen (KP.SIY). Tumors reduced in size upon treatment with immune checkpoint blockade (ICB; anti-CTLA-4 + anti-PD-L1), and by day 14, increased SIY-reactive CD8+ T cells were observed in spleens (particularly the white pulp) and blood, but not in tumors or tdLNs (compared to untreated controls). Single-cell RNA and TCR sequencing, and UMAP analysis of endogenous SIY-reactive T cells identified a pattern of phenotypic clustering that was typical of chronic antigen stimulation. Examining the frequency of each transcriptional phenotype, the researchers found that in both untreated and ICB-treated mice, tdLNs were enriched in transcriptional states associated with early stages of T cell exhaustion, including precursor T cells and progenitor states, while the white pulps of spleens were enriched for intermediate exhausted states, and tumors were enriched for an exhausted 1 state (terminally exhausted cells expressing receptors for IL-12 and IFNγ). Another terminally exhausted population of killer cell lectin-like receptor (KLR)-expressing T cells was also present in the white pulp of spleens, and was generally absent from other tissues. Notably, ICB resulted mainly in the expansion of the intermediate 1 T cell phenotype (resembling tissue-homing effectors), but not intermediate 2 T cells (resembling resident memory precursors), in both spleens and tdLNs.
Most SIY-reactive T cells fell into progenitor, intermediate 1, and exhausted KLR states. To study these cells via flow cytometry, these states could be distinguished as CXCR3+CX3CR1-, CXCR3+CX3CR1+, and CXCR3−CX3CR1+, respectively, by lymphoid tissue-specific gating. In line with the sequencing data, intermediate 1 T cells were most frequent in the white pulp, and were increased upon ICB, primarily in the white pulp. Intermediate 1 T cells also expressed the highest levels of granzyme B. TCF-1 expression, which was highest in progenitor T cells in tdLNs and white pulp, decreased with differentiation towards intermediate 1 and KLR T cells, and in response to checkpoint blockade, but increased among SIY-reactive T cells in the white pulp after ICB.
Next, Morgan, Horton, Bhandarkar, et al. evaluated the TCR repertoire of SIY-reactive T cells and found that clonal expansion was highest in the white pulp. After ICB, TCR diversity decreased, clonal richness increased, and clonal expansion became more even across the tumors, tdLNs, and white pulp. Looking at clonotypes that substantially overlapped between differentiation states, the researchers were able to establish a pathway in which precursor T cells differentiated into progenitor T cells, which differentiated into intermediate 1 and intermediate 2 T cells. Intermediate 1 T cells could then differentiate into either exhausted 1 or exhausted KLR T cells. Additionally, by evaluating clonotypes that straddle differentiation states across various locations, the researchers were able to determine that differentiation from a progenitor to an intermediate 1 state was associated with migration from the tdLN to the spleen, and that differentiation from an intermediate 1 to an exhausted 1 state was associated with trafficking from the spleen to the tumor. In contrast, differentiation from an intermediate 1 to an exhausted KLR state was restricted to the spleen.
Upon ICB, the fractions and clonal sizes of T cells transitioning from intermediate 1 to exhausted 1 T cell states increased substantially. In untreated mice with day 14 tumors, clonal richness peaked at the progenitor and intermediate 1 phenotypes, suggesting that the while the transition from progenitor to intermediate 1 states is efficient, the transition from intermediate 1 to exhaustion isless efficient, but could be enhanced by ICB.
Using an adoptive cell transfer model with SIY-specific TCR-transgenic T cells, the researchers were able to confirm that T cells were primed and began expanding in tdLNs, but then trafficked to spleens, where they acquired an intermediate 1 phenotype and continued to expand. Serial adoptive transfer studies in combination with ICB showed that only cells transferred from the white pulp (and not the tdLN) significantly improved responses to ICB, suggesting that these cells are the primary responders.
To determine whether antigen re-stimulation in the spleen (which has a low antigen density), was required for the efficacy of ICB, the researchers transferred tumor-reactive T cells from the white pulp of KP.SIY tumors to mice bearing KP tumors, and found that they failed to expand. Similar results were observed upon transfer into Batf3-/- mice bearing KP.SIY tumors, which showed reduced T cell expansion in tdLNs and spleens, reduced tumor infiltration, and reduced differentiation from progenitor to intermediate 1 phenotypes. These results suggest that antigen restimulation in the spleen involving dendritic cells was essential to the antitumor efficacy of ICB. However, pulsing splenocytes with SIY prior to transfer (increasing systemic antigen levels) abrogated the expansion of intermediate 1 cells upon ICB, which coincided with increased differentiation from intermediate 1 to exhausted KLR T cells in the white pulp, and reduced tumor infiltration, though these effects could be overcome with ICB.
Finally, the researchers analyzed a multi-cancer patient dataset with matched scRNA and TCRseq data, and identified transcriptional signatures that aligned with those in their mouse data, including a Temra subset that corresponded with exhausted KLR cells. Temra cells were enriched for tumor-specific CD8+ T cells, and while this phenotype was conserved upon entry to the tumor, its frequency there was low relative to peripheral blood, where Temra cells were strongly expanded, suggesting poor tumor infiltration. This was also evident in mouse studies, where the frequency of exhausted KLR T cells was increased in the blood of both control and ICB-treated mice, with only modest increases in expansion in the spleen, suggesting that in mice and humans, this subset expands mainly in the blood, but does not readily infiltrate tumors.
These results map a trajectory of differentiation that is associated with trafficking from the lymph nodes to the spleen, and ultimately to tumors,dependent on an optimal level of antigen restimulation in the spleen. Further, the rate-limiting transition from intermediate 1 to exhausted T cells, which readily infiltrate tumors, could be overcome with ICB. Patterns in patient samples support the clinical relevance of these findings, which help to explain how and where ICB acts.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Duncan Morgan answered our questions.

What was the most surprising finding of this study for you?
It is well established that checkpoint blockade immunotherapy with anti-CTLA-4 and/or anti-PD-(L)1 antibodies can lead to expanded, reinvigorated CD8+ T cell responses against cancer, but exactly which subset of tumor-specific T cells responds to checkpoint blockade has remained unclear. In our study, we analyzed tumor-reactive T cells from three different tissue sites: the tumor, the tumor-draining lymph node, and the spleen. We found that transcriptionally distinct, yet clonally related phenotypes occupied these three tissue sites. Surprisingly, we observed that the spleen-resident population seemed to undergo the greatest degree of expansion in response to checkpoint blockade, and that by dividing, this population gave rise to two distinct transcriptional phenotypes: a conventionally exhausted population, which comprised the majority of tumor-trafficking CD8+ T cells, and a KLR+ exhausted population, which existed in large numbers in the spleen and in circulation, but exhibited limited migration to the tumor.
What is the outlook?
Overall, I think this study fills a small gap in our expanding knowledge about the differentiation of CD8+ T cell clonotypes. Interestingly, the transcriptional phenotypes we describe in our study are very similar to those that have been described in models of chronic viral infection in mice, which remains a key model of the phenomenon of CD8+ T cell exhaustion. However, since these models usually result in an organism-wide infection, including high levels of viral burden in the spleen, they don’t fully recapitulate the tissue site segregation between the tumor, lymph node, and spleen that we observe in most types of cancer. I think it would be interesting to learn more about microenvironmental differences that contribute to the maintenance and selective differentiation of T cell populations across these three tissues. In our study, we performed experiments to suggest that the level of antigen load – which is very high in the tumor, but almost absent from the spleen – is one such factor, but I think it’s likely that many others also contribute. Lastly, the clinical relevance of these findings, such as whether features of splenic, tumor-reactive T cells may help identify potential responders to treatment with immune checkpoint blockade, remain unclear – though we do find evidence in published clinical data for a similar, KLR+ exhausted population that exhibits limited tumor trafficking ability in human patients.
What was the coolest thing you’ve learned (about) recently outside of work?
I recently learned that honeybees, which have brains that are the 0.0002% the size of a human’s, can understand the concept of zero numerosity (specifically, the idea that the quantity of zero is numerically less than a positive quantity) This behavior has only even been observed in a few other animal species (parrot, monkey) and may have been lacking from early human civilizations.
The authors of the paper honor the memory of their friend, colleague, and mentor Brendon Horton, whose contributions and spirit remain sources of joy and of inspiration.




