T cells that maintain a stem-like state play a role in replenishing immune responses long-term, but how these cells are shaped and maintained in the context of antitumor immunity is not fully understood. To investigate this question, Hor et al. combined a multiplex 3D volumetric imaging method with conventional immunological assays. This revealed an important role for cDC1s and PD-1 in the maintenance of stem-like T cells in tumor-draining lymph nodes, and a potential caveat to the use of PD-1-blocking immunotherapies. Their results were published in Nature.
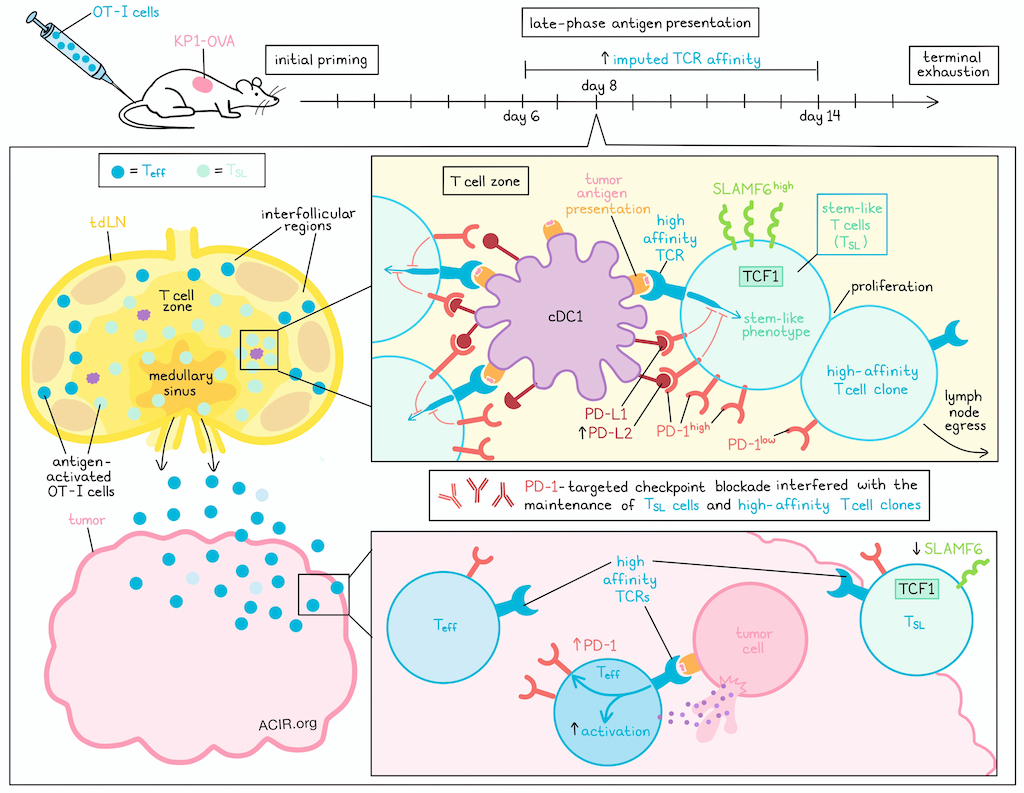
To begin, Hor et al. treated XCR1 reporter mice bearing KP-OVA tumors with adoptive transfer of a low, physiologic level of OT-I cells. Then, using their newly developed 3D tissue staining protocol and computational pipeline for single-cell analysis of large-volume 3D imaging datasets of over 1 million cells of interest, the researchers mapped the spatial distribution and functional states of tumor antigen-specific CD8+ T cells in resected tumor-draining lymph nodes (tdLNs) on day 8.
In tdLNs at day 8 after tumor implantation – a time point that is after the initial priming phase, but before the late exhaustion phase – antigen-activated OT-I cells extensively infiltrated the lymph node T cell zone, interfollicular regions, and medullary sinuses. A small subset of these OT-I cells formed distinct clusters in proximity to XCR1+ cDC1s in the T cell zone, and expressed high levels of both stem-like (TCF-1+ and SLAMF6+) and activation (PD-1+ and BATF+) markers. This finding was somewhat unexpected at this late time point after initial priming, but the researchers confirmed that it was not due to late-arriving naive OT-I cells, and that these T cells were in fact, activated and extensively divided. TCF1+ cells, which the researchers defined as stem-like T (TSL) cells, were localized to the T cell zone, while TCF1- effector T cells were concentrated in the lymph node periphery. TSL cells could also be further classified as either SLAMF6+ (PD-1highSLAMF6high) or SLAMF6- (PD-1intSLAMF6int), the latter of which were less proliferative.
The pattern of increased PD-1 expression in SLAMF6+ TSL cells suggested ongoing antigen signaling events. Investigating this, the researchers evaluated NFAT1 signaling and spatial distribution to show that cDC1s were actively engaging with the TCRs of OT-I TSL cells at this later time point. Similar results were observed across an OVA vaccination model, an endogenous immune model, and an MC38-OVA tumor model.
Investigating whether SLAMF6+ TSL cells could be found outside of the tdLN, the researchers profiled tdLNs, non-tdLNs, spleens, and tumors, and found that while TCF-1+ TSL and TCF-1- Teff cells could be found across all examined tissues, SLAMF6+ TSL cells were exclusive to tdLNs, and expressed higher amounts of the anti-apoptotic protein BCL-2, the stem cell marker CD200, and the stem-like epigenetic profile marker H2AK118Ub. Further, high PD-1 expression outside of the tdLN was only observed in tumors, and while PD-1high TSL cells in tdLNs had the highest SLAMF6 levels, PD-1+ TSL cells in tumors did not show high expression of SLAMF6. Meanwhile, Teff cells in both tdLNs and non-tdLNs had low PD-1 expression. Together these results suggest that SLAMF6highPD-1high TSL cells are retained in tdLNs, while PD-1low TSL and Teff cells egress from tdLNs, and only upregulate PD-1 expression only upon antigen encounter in tumors.
Exploring the functional role of late antigen presentation in tdLNs, Hor et al. utilized XCR1-DTR transgenic mice to enable selective ablation of cDC1s. This showed that late cDC1 depletion (beginning on day 5) significantly reduced OVA-specific CD8+ T cell expansion in tdLNs, which could be mostly attributed to a decline in TSL cells. PD-1high TSL cells were also decreased, and the PD-1 intensity of TSL cells was reduced to that of Teff cells. Using a tetramer-to-CD3 staining ratio to calculate an imputed affinity, further analysis revealed that SLAMF6 expression, antigen-specificity, and high imputed TCR affinity were each correlated with PD-1 expression within the TSL subset. These correlations were only apparent within antigen-draining lymph nodes. These results suggest that cDC1s in tdLNs maintain an expanded population of antigen-specific PD-1+SLAMF6+ TSL cells with high TCR affinities.
Next, the researchers analyzed the imputed TCR affinities of OVA-specific CD8+ TSL and Teff cells from tdLNs collected at different timepoints after tumor induction. While the number of TSL cells remained relatively constant, the average imputed TCR affinity of TSL and Teff subsets increased over time from day 6 to day 14, with Teff cells lagging slightly behind TSL cells, supporting the hypothesis that late antigen-presenting niches in tdLNs selectively enrich high-affinity T cell clones.
Investigating how high-TCR affinity CD8+ TSL cells retain their stemness, despite continued antigen stimulation and proliferation (typically associated with more terminal differentiation), Hor et al. interrogated the role of PD-1 and found that clustered OT-I TSL cells interacting with cDC1s demonstrated polarization of PD-1 towards the T cell–dendritic cell synaptic interface, and that PD-1 on OT-I cells colocalized with PD-L1 on cDC1 cells. Additionally, while PD-L1 was highly expressed among migratory dendritic cells, even at steady state, PD-L2 showed increased expression on cDC1s in the tdLN on day 8. Co-blockade of anti-PD-L1 and anti-PD-L2 revealed that while OT-I cells showed a burst of proliferation after treatment, expression of TCF1 on OT-I cells near cDC1s was reduced and markers of apoptosis were increased, suggestive of activation-induced cell death in the absence of PD-1 signaling. 8 days after checkpoint blockade (anti-PD-L/anti-PD-L2 or anti-PD-1), the average imputed TCR affinity of remaining TSL cells was substantially lower, high-affinity SLAMF6+ TSL cells were reduced, and high-affinity SLAMF6- TSL cells, along with both high and low-affinity Teff cells, were increased. These results suggest that in the absence of PD-1 signaling, high-affinity T cells either differentiate into effector cells or undergo apoptosis, allowing lower-affinity clones to expand as well. Similar results were observed when checkpoint blockade was given later. Further, checkpoint blockade cessation did not reverse the phenotypic shift towards SLAMF6- TSL cells, nor did it replenish the high-imputed-affinity SLAMF6+ TSL cell pool, suggesting the possibility of lasting or even permanent effects.
Together, these results show that in tdLNs, cDC1s maintained a TSL subset by providing sustained antigen presentation for TCR stimulation, while also providing PD-L1 and PD-L2 coinhibitory signals that drove expression of the PD-1 pathway. Importantly, while blockade of the PD-1 axis induced a wave of antitumor immunity, it did so at the expense of high-affinity SLAMF6+ TSL cells. This effect could have long-lasting impacts on immunity, especially in cases where tumors are not cleared upon treatment with PD-1 checkpoint inhibitors.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first author Jyh Liang Hor answered our questions.

What was the most surprising finding of this study for you?
We initially focused on the earlier time points to look at how activated T cells receive their differentiation signals and maintain their stemness. However, on a completely separate project working on developing a 3D tissue-imaging technique, we were surprised by how some activated T cells remained clustered to the antigen-presenting cells, even 7–8 days after the initial priming. Once we found out that it was mostly stem-like cells that received the late antigen signaling, it became an opportunity for us to dissect the mechanisms of how stemness programming relates with antigen presentation.
What is the outlook?
The immune system is a finely tuned system. Inhibitory molecules have evolved to regulate complex fine-tuning of various biological processes, many of which we are only beginning to understand. Our findings show that while checkpoint therapies have achieved remarkable success in treating cancer patients, we still don’t know much about how interference with such finely balanced processes can lead to unanticipated consequences. In attempting to improve the efficacy and safety of immunotherapies, we should also seek to understand how these complex parts interact with one another within tissues, and a systems-level understanding of these processes will be crucial for the field moving forward.
If you could go back in time and give your early-career self one piece of advice for navigating a scientific career, what would it be?
Initial hypotheses are often wrong, at least in my personal experience. However, that does not mean all the effort that went into the thought process and the experiments are wasted. If anything, they can be fresh sources of novel hypotheses, and force you to take on a completely new perspective when interpreting the same set of data. So, I wouldn’t get too fixated when the experimental results do not completely fit the model. I would take it as an opportunity to explore why our current model does not adequately explain the results.




