Overcoming immunosuppression in the tumor microenvironment (TME) is a major challenge in CAR T cell research to improve therapeutic efficacy towards solid tumors. To address some of the CAR-T functionality challenges, Castelli et al. engineered CAR T cells with an authentic IL-9 receptor (IL-9R) to enable IL-9 responsiveness. These CAR-T were assessed in various syngenic murine and human xenograft solid tumor models in a recent publication in Immunity.
The researchers first engineered mouse T cells with a mesothelin-targeting CAR (A03 CAR) and the wild-type IL-9R (CAR-IL9R T cells). Treatment of these cells in vitro with IL-9 resulted in a dose-dependent phosphorylation of STAT1, STAT3, and STAT5. CAR-IL9R T cells showed improved killing capacity, with increased production of IFNγ, TNFα, and IL-10. Furthermore, IL-9 treatment led to a shift toward a stem-like memory phenotype in the CAR-T.
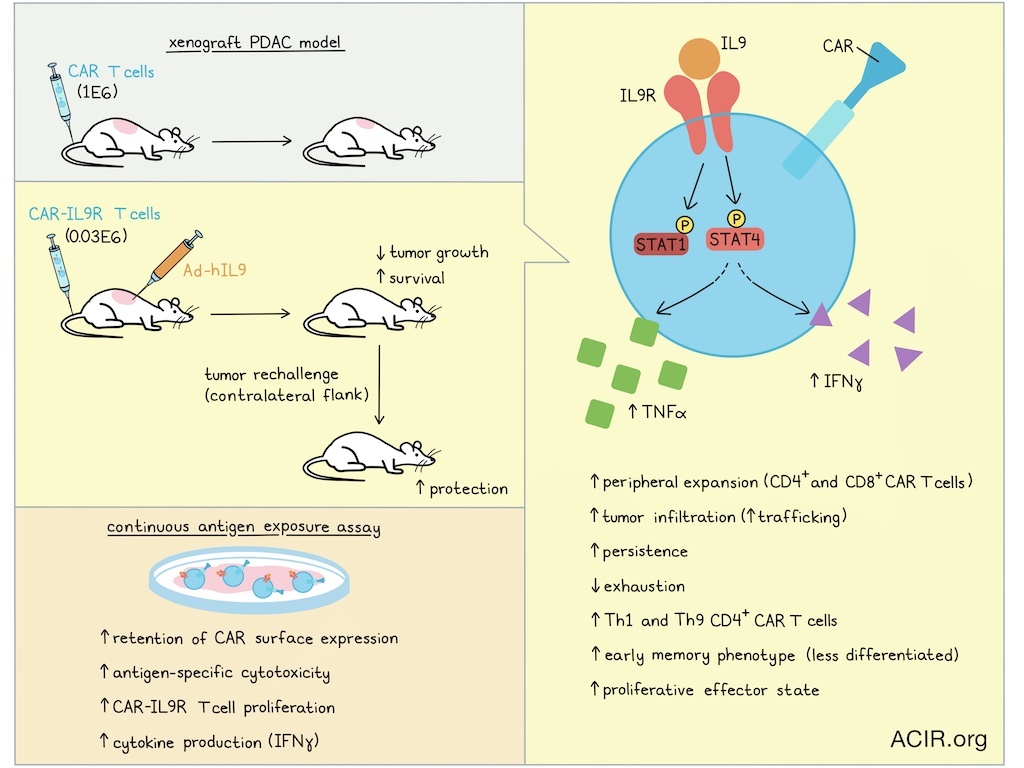
To study in vivo effects, C57/BL6 mice were subcutaneously (s.c.) injected with pancreatic ductal adenocarcinoma (PDAC) PDA7940b cells. T cells from CD45.1+ B6.SJL-PtprcaPepcb/BoyJ mice were used to engineer CAR-IL-9R T cells, which were injected intravenously (i.v.). IL-9 was delivered intratumorally (i.t.) by injection of a replication-deficient adenovirus encoding murine IL-9 (Ad-mIL9) for local production of IL-9. Treatment with CAR-IL9R and Ad-mIL9 improved overall survival (OS) and reduced tumor progression. Tumors and spleens collected on day 5 or 6 of treatment had higher CAR T cell tumor infiltration than controls. This observation suggested improved persistence and trafficking, as no differences in systemic CAR-T cell expansion were detected in the blood. Furthermore, no significant changes in other immune populations were detected in the tumor.
Moving to a human model system, NSG mice were implanted with the human PDAC line AsPC-1. Primary T cells from healthy donors were transduced with a human mesothelin-directed CAR construct (M5 CAR) and IL-9R. Mice were treated i.v. with the CAR-T and received an i.t. injection of Ad-hIL9. Low doses of CAR-IL9R T cells (0.03E6) combined with Ad-hIL9 resulted in improved antitumor efficacy compared to higher doses of control CAR-T (1E6). When mice that were in complete remission after this treatment were rechallenged with AsPC-1 in the contralateral flank on day 90 after treatment, tumors were rejected.
In the human PDAC model system, peripheral CAR-IL9R T cells expanded, persisted, and showed increased cytokine production for up to 10 weeks after treatment. Tumors had higher infiltration of both CD8+ and CD4+ CAR T cells, with a higher proportion of early memory T cells. There was also a decrease in the percentage of PD-1+TIM3+ T cells, and an increase in PD-1-TIM3- and PD-1-LAG3- T cells.
Using a continuous antigen exposure (CAE) assay, in which CAR-IL9R T cells were cocultured with AsPC-1 cells every other day for 10 days, the researchers investigated the effects of repeated antigen stimulation in the presence or absence of IL-9. IL-9-treated CAR-IL9R T showed the best tumor killing capacity, which was antigen-specific and correlated with high production of IFNγ. While control CAR T cells downregulated surface CAR expression, IL-9-treated cells continued to proliferate, and retained CAR expression.
For mechanistic assessment, CAR-IL9R T cells from three donors were subjected to the CAE assay (with or without IL-9), harvested at day 10, and subjected to scRNAseq. Ingenuity pathway analysis revealed the Th1 pathway, IL-20 family signaling, cholesterol biosynthesis, and IL-10 signaling as top-enriched pathways in the IL-9-treated CAR-T.
Based on these results, the researchers investigated the role IL-10 plays in the phenotype and function of CAR-IL9R T cells. NSG mice were engrafted with PC3-PSMA prostate tumor cells and treated i.t. with Ad-hIL9, and i.v. with CAR-T (targeting PSMA, coexpressing a dominant negative TGF-βRII and IL-9R). In this model, the CAR-IL9R T cells plus IL-9 treatment also resulted in reduced tumor progression and improved OS, with increased numbers of peripheral CAR-T compared to controls. IL-10 neutralization did not affect antitumor efficacy or blood CAR T cell counts, suggesting that IL-10 was not a major driver of the enhanced functionality.
The researchers then performed clustering analysis on the scRNAseq data. In the IL-9-treated group, a higher proportion of cells were found in effector clusters, with an increase in the CD4+ activated and GNLY++ effector clusters, while the group without IL-9 treatment had more CAR-T in the CD8+ exhausted and GZMA++ cytotoxic clusters. Differential gene expression analysis showed that IL-9 treatment in CD8+ CAR-IL9R T cells increased transcription factors, chemokine-related genes, markers of naive/memory CD8+ T cells, and cytotoxicity-related genes. In CD4+ CAR-IL9R T cells, IL-9 treatment increased expression of chemokine-related genes, markers of naive/memory T cells, proliferation-associated genes, and cytotoxicity-related genes. Gene set enrichment analysis revealed increases in expression of genes related to interleukin signaling pathways in CD4+ and CD8+ CAR-T, and within the CD4+ population, there was an increase in Th1 and Th9 cells.
Assessing CAR T cell trajectories over pseudotimes identified 6 trajectories, of which, four differed between CAR T cells treated with IL-9 and those exposed to mock conditions. In CD8+ T cells, IL-9 enhanced progression from naive to central memory and effector states, while progression to exhausted/dysfunctional states was reduced. In CD4+ T cells, trajectories toward proliferative states were promoted. These data were confirmed by RNA velocity analysis, which also showed a pattern of movement away from terminal states, and toward less differentiated phenotypes and more proliferative effector states.
Investigation of the regulatory interactions between transcription factors and genes showed a strong enrichment in STAT4 activity in the CAR-IL9R T after IL-9 treatment, which has not previously been linked to IL-9 signaling. The researchers validated this finding at the protein level, showing robust phosphorylation of STAT4. STAT4 deletion using CRISPR-Cas9 reduced IFNγ and TNFα production in IL-9-treated CAR-IL9R T cells. These cells had a slight increase in memory markers, and a limited reduction of in vitro cytotoxicity, suggesting STAT4 may contribute to the IL-9-mediated effector functions.
Therefore, IL-9 signaling in CAR-T in the TME positively impacts T cell fate, resulting in less differentiated states and reduced T cell exhaustion. Given that this modification improved antitumor activity, CAR T cell expansion, and persistence in solid tumor models, it might help overcome some of the major obstacles to clinical CAR-T treatment of solid tumors.
Write-up by Maartje Wouters, image by Ute Burkhardt
Meet the researcher
This week, first author Sofía Castelli answered our questions.

What was the most surprising finding of this study for you?
The most surprising finding for me was how dramatically IL-9 signaling amplified CAR T cell potency. We found that very low doses of IL-9-engineered CAR T cells were sufficient to clear tumors in vivo, which was far beyond what we anticipated. Even more striking, these cells were not only more effective, but also far more persistent, remaining in circulation long after conventional CAR T cells contracted. Our in vitro dysfunction model mirrored this behavior; IL-9 signaling essentially reprogrammed CAR T cells toward effector, memory, and proliferative states, rather than exhaustion. This level of fate redirection was completely unexpected.
What is the outlook?
A key next step is to test IL-9-signaling CAR T cells across additional solid tumor models and in more clinically relevant systems, such as humanized or patient-derived models, before we can eventually move this therapy into the clinic. It will also be important to further investigate the molecular pathways that underlie the improved phenotype. From an engineering perspective, developing tunable or inducible IL-9 circuits and exploring combinations with complementary therapies could further enhance efficacy. Together, these studies will help define the broader applicability of IL-9-signaling CAR T cells and their potential for clinical translation.
If you could go back in time and give your early-career self one piece of advice for navigating a scientific career, what would it be?
I would tell my early-career self not to be discouraged by uncertainty or by experiments that don’t work as planned. Those moments are a normal part of research and often lead to better questions. I’d also remind myself to step back occasionally and keep the broader scientific goals in view, rather than getting lost in day-to-day setbacks. Some of the most meaningful progress comes from exploring ideas that fall slightly outside the obvious or expected, so staying open to those directions is important.




