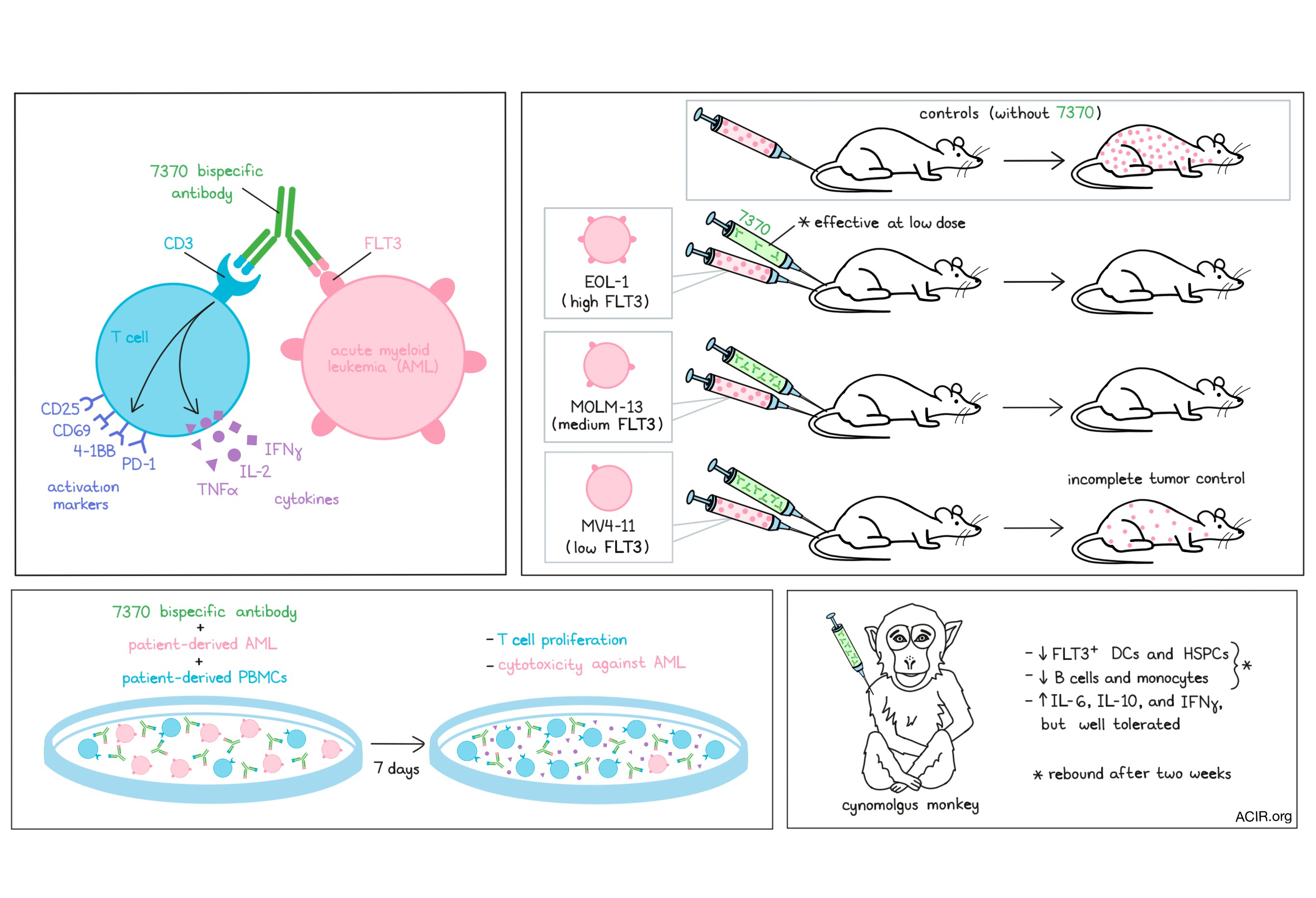
In an effort to address the significant unmet clinical needs in the treatment of acute myeloid leukemia (AML), Yeung and Krishnamoorthy et al. developed a novel bispecific antibody that targets both CD3 on T cells and FLT3 on AML cells. FLT3 was selected as a strong potential target antigen, given its upregulated expression on AML blasts in a high percentage of patients, its role as an oncogenic driver, and its absence from most healthy cell types, besides dendritic cells (DCs) and hematopoietic stem and progenitor cells (HPSCs), which typically express FLT3 at low levels. The researchers optimized and tested their bispecific antibody, deemed 7370, and their results were recently published in Molecular Therapy.
In order to design an optimized bispecific antibody, Yeung and Krishnamoorthy et al. screened a panel of human antibodies and selected representative antibodies targeting different domains of human FLT3 to be incorporated into full-length bispecific antibodies co-targeting CD3. In an in vitro cytotoxicity assay against the AML cell line EOL-1, bispecific antibodies targeting the fourth (the second-most membrane proximal) and fifth (the most membrane proximal) domains were most effective. In NSG mice bearing subcutaneous EOL-1 AML tumors, the bispecific antibody targeting the fourth domain clearly showed greater antitumor efficacy, despite not having the highest affinity, which may reflect a more optimal geometry of the synapse. Several antibody clones targeting domain 4 were then tested, and the top clone was selected to be incorporated into a bispecific antibody. The resulting full-length FLT3/CD3-targeting bispecific antibody, 7370, was able to specifically bind to recombinant FLT3 and CD3 proteins, human T cells, and the AML cell lines EOL-1 (high FLT3 expression) and MV4-11 (low FLT3 expression).
In short-term cytotoxicity assays using healthy donor T cells and EOL-1 or MV4-11 AML cell lines, 7370 induced nearly complete lysis of both EOL-1 or MV4-11 AML cells at an effector-to-target (E:T) ratio of 1:1. Substantial target cell lysis of both EOL-1 or MV4-11 AML cells was observed at an E:T ratio as low as 1:20, though less lysis was induced in MV4-11 cells, suggesting that antigen density affects the efficacy of 7370. In the presence of FLT3+ AML blasts (but not FLT3- cell lines), 7370 potently induced T cells to express activation markers (CD25, CD69, 4-1BB, and PD-1) and produce IFNγ, TNFα, and IL-2, consistent with TCR-driven T cell activation. Evidence of T cell activation was transient, and disappeared (except for CD25) when tumor cells were eliminated.
To test the efficacy of 7370 in vivo, Yeung and Krishnamoorthy et al. engrafted NSG mice with either EOL-1 (high expression of wild-type FLT3), MOLM-13 (medium expression of mutant FLT3), or MV4-11 (low expression of mutant FLT3) cell lines to generate orthotopic xenograft models of AML. Mice were injected with previously activated and expanded human T cells, followed by a single injection of 7370 at various doses. 7370 was able to protect all mice against EOL-1 or MOLM-13 for at least 40 days post-implantation, though a higher dose was required to achieve complete control over MOLM-13 growth. A single dose of 7370 reduced the growth of MV4-11 cells, but was not sufficient to eliminate them entirely, suggesting that low FLT3 expression could be a barrier to antitumor efficacy.
Having shown that 7370 can induce antitumor efficacy in mice, the researchers went on to explore its potential clinical relevance. To this end, they tested PBMCs from 5 patients against their own AML blasts in the presence of 7370 in vitro. The E:T ratio in these samples ranged from 1:25 to 1:64, and expression of FLT3 on AML cells varied. Despite the low E:T ratios, 7370 induced T cell proliferation in a dose-dependent manner, as well as substantial cytotoxic responses from T cells in 4 out of 5 samples. In 2 out of 4 samples, AML cells were over 80% depleted by day 7. The dose of 7370 required was significantly higher than with T cells from healthy individuals, possibly reflecting a lower T cell quality or presence of inhibitory checkpoints in patient-derived T cells.
In order to evaluate potential hematological toxicities associated with 7370, Yeung and Krishnamoorthy et al. tested their bispecific antibody in healthy cynomolgus monkeys. After showing that 7370 was cross-reactive to cynomolgus FLT3, and that, like in humans, FLT3 was expressed on DCs in the blood and on CD34+ HSPCs in the bone marrow, four cynomolgus monkeys were treated with two doses of 7370, administered one week apart. One week after the initial dose, FLT3+ DCs (CD1c+ and CD123+) and FLT3+CD34+CD38+ HSPCs were nearly or completely depleted. Two of the monkeys were observed for a two-week recovery period, during which levels of FLT3+ DCs and HSPCs were restored roughly to baseline levels. Other immune cell subsets, including B cells and monocytes, were also depleted following treatment with 7370, but these too were restored over the two-week recovery period. Evaluation of cytokine release patterns showed that 7370 increased expression of IL-6, IL-10, and IFNγ after first, but not the second, dose. Cellularity was minimally to mildly decreased in the bone marrow, consistent with the depletion of FLT3+ cells, and cellularity was minimally to moderately increased in spleens and axillary lymph nodes, consistent with T cell activation. One monkey experienced low-grade adverse events, but overall, 7370 was well-tolerated.
Overall, evidence from mice, patient samples, and monkeys, shows that 7370 can effectively redirect T cells to target and kill FLT3+ target cells, in a dose-dependent and antigen density-dependent manner. 7370 was well-tolerated and its effects on the immune system appeared to be reversible, suggesting that 7370 has the potential to be safe and effective against AML in a clinical setting.
by Lauren Hitchings
Meet the researcher
This week, first author Yik Andy Yeung answered our 3 questions.
What prompted you to tackle this research question?
We would like to develop a therapy that can broadly treat AML with durable response. AML is a heterogeneous disease. Continual understanding of the underlying disease mechanism did lead to the development of novel targeted therapies, however, such therapies only benefit subset of patients and the disease often relapses. Meanwhile, T cell redirection therapy (Blinatumomab: CD19-CD3 for ALL) showed great promise in delivering long-term responses. However, proper target selection is critical for T cell redirection therapy to work, as broad expression of the target receptor in healthy tissues can severely limit the therapeutic efficacy. Therefore, we sought to identify a target receptor on AML that is critical for their survival and differentially overexpressed as compared to normal cells. That’s how we arrived at FLT3 as a T cell redirection target for AML.
What was the most surprising finding of this study for you?
One of the surprise findings was on the engineering of the candidate molecule. Multiple studies suggested that for T cell redirection therapy, bispecific antibody targeting the membrane proximal region of the target receptor can help bring the T cell closer to the cancer cell, thereby mediating more effective killing. However, when we tested the efficacy of bispecific antibodies targeting different regions of the FLT3 receptor, we found that the most optimal domain to target is not the most membrane proximal domain, rather it is the adjacent domain further away from the membrane. This implies that proper screening of the binding epitope on the target receptor is very important for the engineering of the bispecific antibody.
What was the coolest thing you’ve learned (about) recently outside of work?
Recently, I sat in on my son‘s karate lesson. My son was asked by the sensei (teacher) to demonstrate certain skill sets for the younger apprentice. Watching him performing the forms and steps confidently in the class suddenly reminded me of him at an earlier age, when he could not properly do these steps. However, through the help of sensei and practices, he managed to learn the steps over time. Beside being proud of him, I am very grateful for the sensei’s passion and patience in teaching him. It is such a blessing in life to have a teacher/mentor with such passion, knowledge, and patience.




