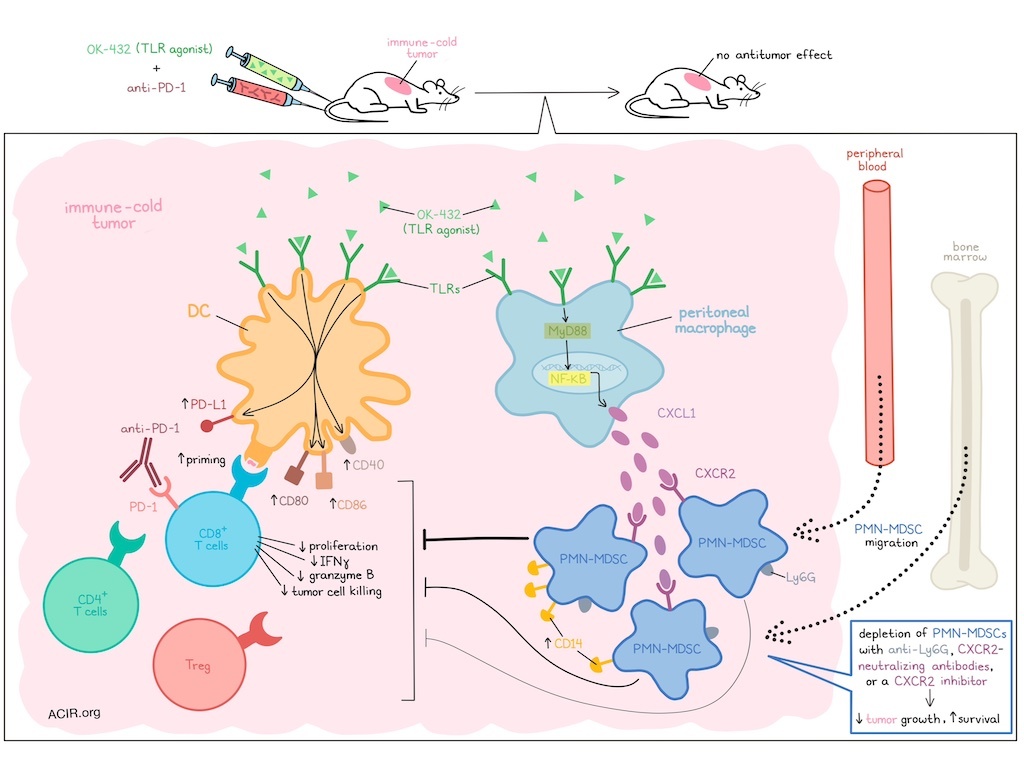
The activation of an immune response involves a system of checks and balances. While immunotherapies can be used to tip the scales, they are not always effective, as resistance mechanisms may act to counter desired responses. In research recently published in Science Translational Medicine, Nishinakamura and Shinya et al. found that a combination of the TLR agonist OK-432 plus PD-1 blockade failed to induce antitumor responses in immune-cold tumor models. Upon further investigation, they were able to attribute this resistance to an unintended co-activation of PMN-MDSCs, which could be overcome through PMN-MDSC depletion.
To begin, Nishinakamura and Shinya et al. evaluated OK-432 – an agonist for multiple TLRs – against human DCs derived from both healthy donors and patients with gastrointestinal cancers, and found that it effectively induced DC maturation, enhancing expression of CD86, CD80, CD40, and PD-L1. Functionally, OK-432 also improved DC priming of peptide-specific CD8+ T cells, and this effect was further enhanced with the addition of anti-PD-1.
Moving into mouse models, the researchers investigated the combination of OK-432 and anti-PD-1 in a variety of cold tumor models, including LL/2 Lewis lung carcinoma, CT26 colon carcinoma, and CT26-NY-ESO-1. While the combination treatment slightly inhibited tumor growth in the CT26 model and slightly prolonged survival in the CT26-NY-ESO-1 model, there was no advantage over either monotherapy, and no advantage at all in the LL/2 model.
Investigating this on an immunological level, the researchers evaluated tumors by flow cytometry and found that while OK-432 effectively upregulated CD80 and PD-L1 on DCs, CD8+ T cells, CD4+ T cells, and Tregs were all unexpectedly reduced in frequency. Bulk RNAseq, gene set enrichment analysis, and CyTOF/UMAP analyses each suggested that MDSCs, and particularly PMN-MDSCs, were upregulated in tumors. These results were further confirmed experimentally, with flow cytometry showing an increase in Ly6G+ PMN-MDSCs, but not Ly6C+Ly6G- M-MDSCs in tumors upon treatment with OK-432. Further, CD8+ T cells cocultured with FACS-purified PMN-MDSCs showed reduced proliferation, reduced production of IFNγ, and limited capacity for cell killing.
Looking deeper into the mechanism by which PMN-MDSCs accumulated in tumors, the researchers found that Ly6G+ cells were reduced in the peripheral blood and bone marrow of mice 24 hours after OK-432 administration, suggesting that PMN-MDSCs likely migrated from these locations to tumors upon with treatment OK-432. Evaluating cytokines and chemokines that might mediate this migration, the researchers noted upregulation of IL-6 and CXCL1 in tumors. Evaluation of IL-6 via analysis of STAT3 activation ruled out a role for IL-6 in the recruitment of PMN-MDSCs. However, the increase in CXCL1 production was found to originate from peritoneal macrophages (but not tumor cells) in the TME and was dependent on TLR–MyD88–NFκB signaling. Further, CD45+CXCR2+ cells in the TME highly expressed Ly6G after OK-432 treatment, suggesting that PMN-MDSCs were likely recruited via the CXCR2–CXCL1 axis.
To determine how different tumors might respond to OK-432, Nishinakamura and Shinya et al. investigated its effects on 10 different cancer cell lines. Bulk RNA sequencing along with UMAP and clustering analysis were performed, and different tumor cell lines were grouped based on their haplotypes and levels of immune infiltration. While the top three most highly immune-infiltrated lines (MC38, MC38-OVA, and EMT6) responded to a combination of Ly6G+ cell depletion (using anti-Ly6G) and anti-PD-1, the remaining cell lines, including LL/2, CT26, and CT26NY-ESO-1, did not. However, when the researchers tested a triple combination of OK-432 plus anti-PD-1 plus Ly6G+ cell depletion (using anti-Ly6G or CXCR2-neutralizing antibodies), treatment effectively inhibited tumor growth and prolonged survival compared to controls, monotherapies, and double combination therapies in immune-cold models.
Looking within the PMN-MDSC subset induced by OK-432, the researchers found that in CT26-NY-ESO-1 tumors, treatment induced a particularly suppressive subset of CD14high PMN-MDSCs. However, triple combination therapy with the anti-Ly6G reduced these Ly6G+CD14high PMN-MDSCs, along with Ly6G+CD14intermed and Ly6G+CD14- PMN-MDSCs. Use of CXCR2-neutralizing antibodies was not as effective as anti-Ly6G in the triple combination, which may be due to differences in the potency of the antibodies towards this highly suppressive subset. Similar results were observed in an LL/2 tumor model. Further, CD8+ T cells, but not CD4+ T cells or NK cells in the LL/2 tumor model were required for the antitumor efficacy of triple combination treatment. Triple combination treatment also increased antigen-specific CD8+ T cells and their effector functions (granzyme B and IFNγ production) compared to OK-432 or anti-Ly6G alone. A CXCR2 inhibitor could also be used in place of anti-Ly6G or CXCR2-neutralizing antibodies as a means of inhibiting PMN-MDSCs, showing similar antitumor effects when used in triple combination with OK-432 and anti-PD-1.
Finally, Nishinakamura and Shinya et al. investigated whether their results in mice were relevant in patients by evaluating patients with lung cancer who were treated with OK-432 to control pleural effusion, as it is approved for this use in Japan. In these patients, concentrations of CXCL1 were increased in the pleural effusion after treatment, as were levels of CD14+ cells among CXCR2+CD45+ cells, and expression of LOX1 (a marker of human PMN-MDSCs) on a per cell basis, which aligns with an increase in PMN-MDSCs in response to treatment.
Overall, these results suggest that while TLR agonism may activate DCs and enhance their capacity for T cell priming, it also recruits immunosuppressive PMN-MDSCs, limiting efficacy alone or in combination with anti-PD-1 in immune-cold tumor models. However, depletion of PMN-MDSCs could be used to restore the efficacy of TLR agonism plus anti-PD-1, shifting the balance towards immune activation and antitumor efficacy.
Write-up and image by Lauren Hitchings





