Pediatric patients with diffuse intrinsic pontine glioma (DIPG) or other H3K27M-mutated diffuse midline gliomas (DMGs) have few treatment options, and a lethal prognosis. In an effort to fight these deadly cancers, Majzner and Ramakrishna et al. recently began a phase I clinical trial testing the use of CAR T cells directed at GD2, which is highly expressed in H3K27M-mutated DMGs, and only minimally expressed in normal brain tissue. Encouraging early results for the first four treated patients were recently published in Nature.
Based on previous results, Majzner and Ramakrishna et al. anticipated the development of tumor inflammation-associated neurotoxicity (TIAN) following treatment with GD2-CART. To mitigate the risks associated with TIAN, the researchers planned for patients to have an Ommaya catheter placed in order to monitor intracranial pressure. They also instituted a TIAN toxicity management algorithm, which incorporated the removal of cerebrospinal fluid (CSF) through the Ommaya catheter, hypertonic saline, anti-cytokine agents, and corticosteroids. In the patient selection process, patients with bulky thalamic or cerebellar tumors were excluded.
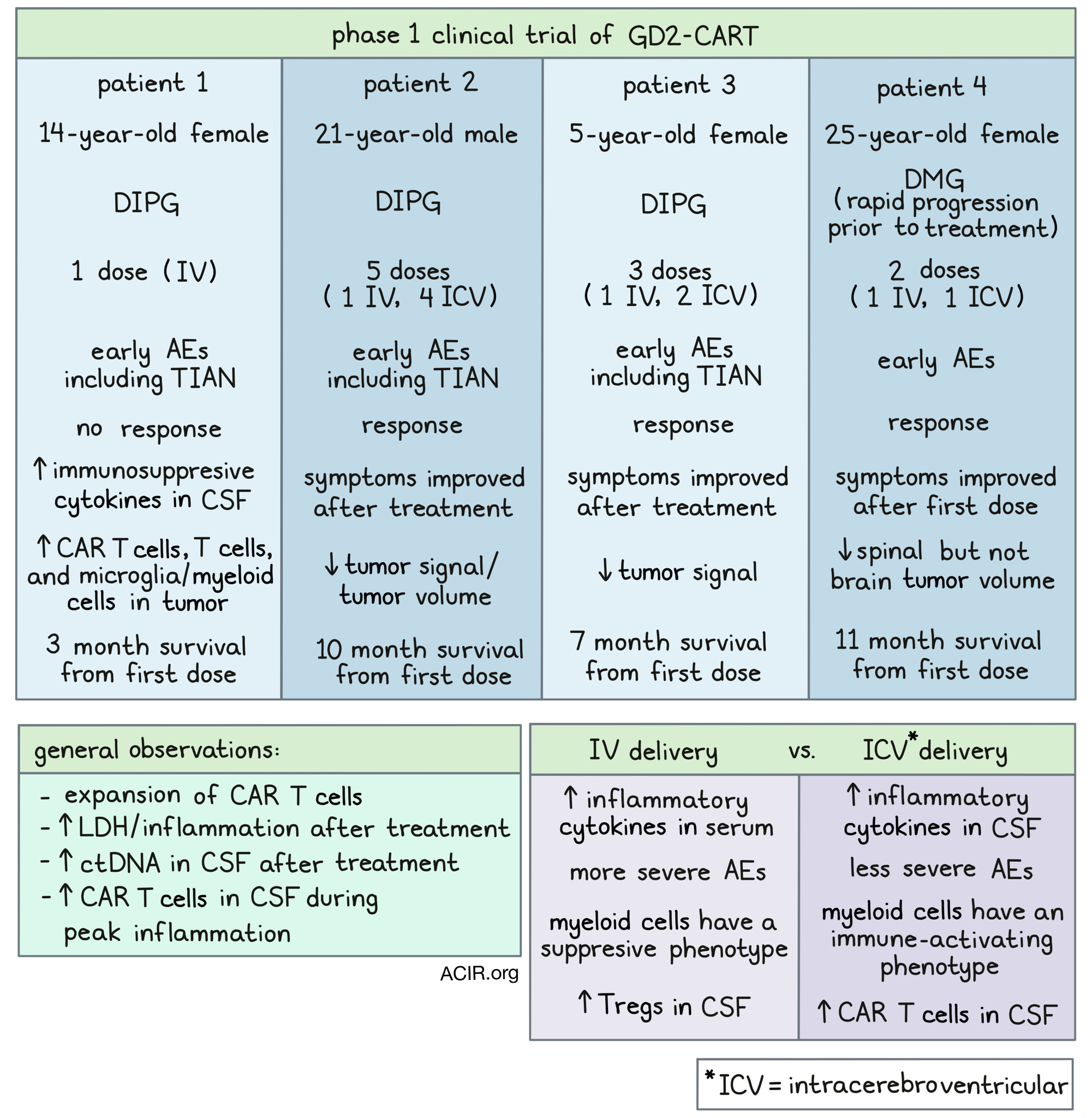
The first patient in this clinical trial was a 14-year-old female with DIPG. Following intravenous (IV) treatment with GD2-CART, this patient experienced early AEs that could be managed with intervention. Unfortunately, this patient did not respond to treatment; one month after the administration of GD2-CART, this patient’s disease progressed, and 2 months later, she passed away. Postmortem evaluation of the tumor and normal brain tissue confirmed increased GD2 expression in the tumor tissue. It also revealed infiltration of lymphocytes, including CAR T cells, into the tumor (but not normal brain tissue), which is not characteristic of DIPG. Microglia/myeloid infiltration and high levels of immunosuppressive cytokines in the CSF were also observed in this patient, which could have contributed to her lack of response. Further, the CAR T cell product showed evidence of increased tonic signaling, which could have also contributed.
The second patient in this trial was a 21-year-old male with DIPG. This patient was experiencing neurological symptoms that limited a variety of sensations and functions, including walking and eating. Following IV treatment with GD2-CART, this patient experienced low-grade AEs and temporary worsening of symptoms, consistent with TIAN affecting function. However, by two weeks after treatment, the patient’s symptoms had improved significantly, and some previously impaired functions were restored. By one month after treatment, the patient tested near to normal on a neurological exam. An MRI showed areas of improved T2/FLAIR signal, and slightly decreased tumor volume. These clinical and radiographic improvements lasted 2-3 months before the tumor progressed and symptoms returned.
This patient then received a second, higher dose of GD2-CART, this time delivered intracerebroventricularly (ICV) via the Ommaya reservoir. Again, the patient experienced manageable AEs and transient TIAN-related worsening of symptoms. After two weeks, the patient’s tumor volume had reduced, his neurological exam was almost normal, and his physical functionality and quality of life had greatly improved. After the data cutoff, the patient went on to receive three additional ICV infusions of GD2-CART, but ultimately passed away 10 months after the first infusion due to an intratumoral hemorrhage – a common occurrence in patients with DIPG.
The third patient in this trial was a 5-year-old female with DIPG. She was experiencing a range of neurological symptoms, which limited a variety of normal bodily functions and interfered with her quality of life. Following IV treatment with GD2-CART, this patient experienced early, low-grade AEs, which could be managed with interventions, and transient TIAN-associated worsening of symptoms. By two weeks post-infusion, many of the patient’s symptoms had improved, and continued to improve at one month and six week follow-ups. At one month, an MRI showed decreased T2/FLAIR signal abnormality in the midbrain.
By three months after the initial dose, the patient’s improving clinical symptoms had plateaued and disease progression began. The patient was re-treated with a higher dose of GD2-CART delivered ICV. The patient experienced minimal AEs, which did not require intervention. Within a week, the patient experienced improvements on one side of the body, but functions on the other side progressively worsened. After data cutoff, the patient received one more infusion, but her disease continued to progress and she died 7 months after the first infusion.
The fourth patient in this trial was a 25-year-old female with spinal DMG. After enrollment and during the cell manufacturing stage, the patient experienced rapid tumor progression, tumor spreading, and dramatic worsening of symptoms and quality of life. An emergency duraplasty was performed, and the patient was treated with corticosteroids. Despite no longer meeting the criteria for this study, the patient was given an IV infusion of GD2-CART on a single-patient compassionate use eIND. The patient experienced serious, but manageable AEs. Despite rapid tumor progression prior to treatment, many of the patient’s symptoms improved, and imaging showed over 90% reduction in spinal cord tumor volume by day 47, though brain metastasis did not improve. By day 75, the patient’s brain and spinal cord tumors had progressed. The bulkiest tumor in the brain was partially resected, and while the tumor sample showed robust GD2 expression, few infiltrating T cells and only minimal levels of GD2-CAR T cells (by qPCR) were found.
Following the administration of a second dose of G2D-CART, delivered ICV, this patient again experienced serious, but manageable AEs, including encephalopathy. Again, tumor regression was observed, with an over 80% decrease in spinal cord tumor volume, along with reduction in select areas of brain disease. Unfortunately, clinical improvement was not observed with this dose, and within two months, the spinal and brain disease progressed. The patient passed away 11 months after the first dose.
Samples of serum and CSF taken from these patients throughout the course of treatment showed that in all patients, following IV or ICV delivery, CAR T cells expanded and LDH levels increased, consistent with increased inflammation. At times of peak inflammation, more cell-free tumor DNA and more CAR T cells were detected in CSF in evaluable patients.
Comparing IV and ICV administration, the researchers found that inflammatory cytokines were higher in the CSF following ICV delivery, while they were higher in serum following IV delivery. This is consistent with more severe AEs observed following IV doses. In one evaluable patient, more CAR T cells were present in the CSF following ICV versus IV administration. The researchers also identified a population of myeloid cells with an immune-activating signature in CSF during peak inflammation following ICV, while immunosuppressive myeloid subpopulations dominated in the CSF following IV administration. Further, increased Tregs were identified in CSF after IV versus ICV CAR T cell administration.
Overall, this early clinical data shows promise for the use of GD2-CART to treat pediatric and young patients with H3K27M-mutated DMGs, and provides early insight into how to improve this treatment protocol. This trial will continue on with additional patients, advised by the experiences and results observed in these early participants.
Write-up and image by Lauren Hitchings
Meet the researcher
This week, first co-authors Robbie Majzner and Sneha Ramakrishna answered our questions.

What prompted you to tackle this research question?
RM: As pediatric oncologists, DIPG/DMG is the absolute worst diagnosis we deliver to families. There is no cure for this universally fatal disease, and few or no active therapies. Families are left to grapple with the diagnosis and watch their child suffer and ultimately die from this awful disease. So when in 2018, we found that GD2, a well known target in pediatric oncology, is highly expressed on DIPG, and that GD2 CAR T cells are highly active in mouse models, we decided to bring this to the clinic as quickly as possible. We were in the fortunate position that we were already planning trials using a GD2 CAR for other childhood cancers, as several groups before us, including those at Baylor and University College London, had shown that GD2 CAR T cells were safe in humans. So we fortunately were able to significantly speed up the process.
SR: We are driven by the incredible bravery of our patients, who enrolled on this phase 1 clinical trial and allowed us to learn from them in order to bring hope to future patients. We wanted to ensure that we learned the most that we could – both clinically and scientifically – to honor the courage of these individuals. As part of the trial design, I led our team to establish a standardized correlative platform that allowed for real-time processing of patient peripheral blood and cerebrospinal fluid samples at clinically relevant timepoints. Using these samples, we developed a rich correlative database, identifying signals of CAR T cell activity and potential contributors to CAR T cell failure. By understanding the biology of GD2 CAR T cells in these patients, we are identifying ways to improve CAR T cell activity and efficacy. This paper also shows that collaborative science and thoughtful correlative platform design are essential to the continued iterative advancement of CAR T cell therapies. None of this would be possible without the bravery of our patients. We hope to build on these lessons learned to carve a path to cure.
What was the most surprising finding of this study for you?
RM: First, that the GD2 CAR T cells worked. Even though responses weren’t durable, we were amazed to see regressions in this aggressive cancer – one of the first times CARs have really worked for solid tumors. It was so gratifying to see results from the lab validated in humans. And second, that we were able to successfully re-treat patients with multiple infusions. With CD19 CAR T cells in leukemia/lymphoma, re-treatment rarely works, but in this trial, we have been able to re-treat patients multiple times by delivering cells directly into the CNS. The CARs continue to work with each infusion, resulting in even more exciting results to be presented soon!
SR: By far the most surprising thing about this study is that our patients had significant clinical improvement of their neurologic deficits within weeks of treatment with GD2 CAR T cells. This clinical response aligned with evidence of CAR T cell activity in the correlative samples we collected from our patients. From the correlative findings, I was surprised by the pronounced myeloid suppressive populations identified in patients receiving CAR T cells intravenously (IV) and in late timepoints following intracerebroventricular (ICV) CAR T cells. These suppressive cells may explain why our patients had limited durability of response and required repeat CAR T cell administration. Remarkably, these second infusions were able to provide patients with repeated clinical benefit. Correlating with this response, we identified an activating myeloid signature unique to the peak inflammatory time point after ICV CAR T cell infusion. These myeloid cells seem to be central to the ability of CAR T cells to function, which gives us the opportunity to potentially target them to improve the durability of CAR T cell activity.
What was the coolest thing you’ve learned (about) recently outside of work?
RM: Kids will hike if they are with other kids. My children complain non-stop if it’s just us, but when we are with other families, they just forget to complain and actually enjoy hiking!
SR: When I was a kid, I used to love imagining all sorts of things. It was part of the joy of a life without responsibilities or worries. Parenting during the last two years of this pandemic has been incredibly difficult. Despite these challenges, my daughter never lets me forget my inner child. In fact, I have been able to re-ignite my childhood imagination, traveling to Saturn on Saturday, and re-discover my love of art, making dinosaur Valentine’s Day decorations on Sunday. Thank goodness for the unbalanced balance of family. Oh, and I’ve also learned that we don’t talk about Bruno.




