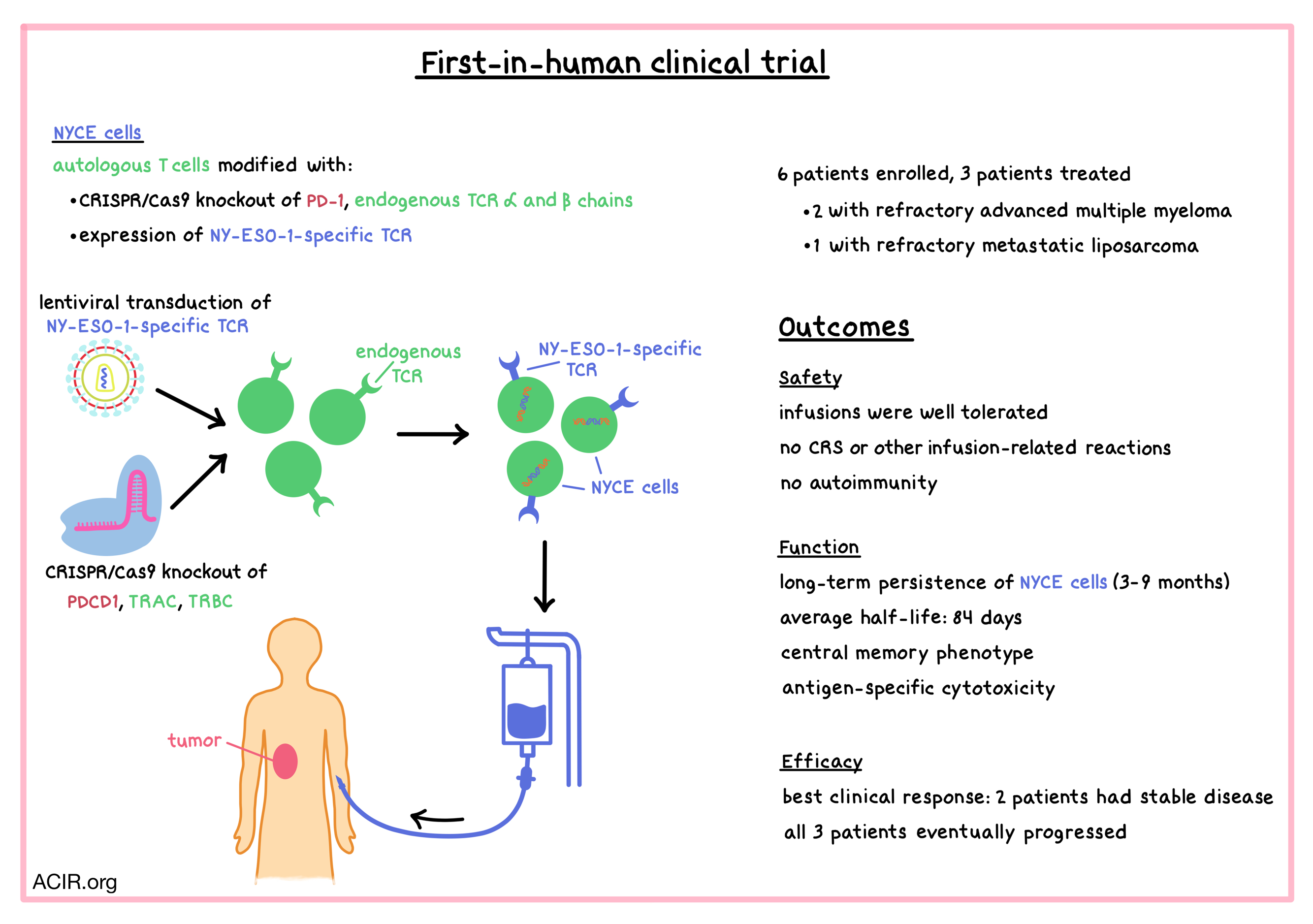
Aiming to improve the safety and efficacy of engineered T cells for the treatment of human cancers, Stadtmauer and Fraietta et al. utilized CRISPR-Cas9 genome editing to knock out PD-1 (PDCD1) and the endogenous TCR α and β chains (TRAC and TRBC) in autologous T cells that also expressed an exogenous TCR targeting the cancer-specific antigen NY-ESO-1. The engineered T cells were tested in a first-in-human clinical trial, and the results were recently published in Science.
Patient T cells were harvested and modified by Cas9/guide RNA complexes (delivered by electroporation) and an HLA-A2*0201-restricted TCR specific for the SLLMWITQC peptide in NY-ESO-1 and LAGE-1 cancer-testis antigens (delivered by lentiviral transduction). The genetically edited autologous T cells were called “NYCE” (NY-ESO-1 transduced CRISPR 3X edited cells). NYCE cells were successfully created for four out of six enrolled patients. One of those four patients experienced rapid disease progression and became ineligible for infusion. Thus, three patients (two with refractory advanced multiple myeloma, and one with refractory metastatic liposarcoma) were infused with the NYCE product. Prior to infusion, patients were pre-treated with lymphodepleting chemotherapy.
Before administration of the engineered T cells to patients, NYCE cells were analyzed in vitro for editing efficiency and potency. Analysis of the CRISPR-Cas9 editing efficiency showed that most mutations were on-target (albeit with efficiencies of 45% [TRAC], 15% [TRBC], and 20% [PDCD1]), and very few off-target mutations were detected. Chromosomal translocations were observed in all manufactured NYCE products, but they declined in frequency in patients over time, suggesting that the translocations did not provide any cell growth advantage. In a co-culture with HLA-A2+NY-ESO-1+ Nalm-6 leukemia cells, NYCE cells efficiently killed target cells in an antigen-specific manner. Consistent with preclinical experiments, T cells with endogenous TCR knockout were more cytotoxic than T cells that retained their endogenous TCR.
NYCE infusions were well tolerated, and no cytokine release syndrome or other side effects related to infusion were observed in any of the three treated patients. The engineered T cells peaked at high levels shortly after infusion and persisted for a long time in all three patients (from three to nine months post infusion), indicating stable engraftment. The average half-life of NYCE cells was 84 days, which was strikingly higher than the ~1 week half-life of NY-ESO-1 engineered T cells reported in other clinical trials. Biopsies showed that NYCE cells trafficked to the tumor (or in the case of multiple myeloma, the bone marrow) at levels close to those in the blood. Despite successful engraftment of PD-1- NYCE cells, no patients exhibited evidence of autoimmunity. While healthy donors usually develop antibody and T cell responses to Cas9, the three treated patients did not – possibly due to low levels of Cas9 in the infused product, or the patients’ immunodeficiency due to previous treatments/lymphodepletion. Lack of reactivity to Cas9 was consistent with the prolonged persistence of the infused product.
The researchers analyzed the transcriptomic evolution of NYCE cells over time in one patient who showed signs of tumor regression. NYCE cells were recovered from the blood at day 10 and at day 113 (~4 months) after infusion. Single-cell RNA sequencing showed a decline in the frequency of gene-edited T cells over time (regardless of whether the cells had a transduced TCR). Most of the decline occurred in the first 10 days, with the frequency of gene-edited cells holding generally stable between 10 days and 4 months post infusion. Cells in the manufactured product had mutations in one, two, or all three targeted sequences. Approximately 25% of engineered T cells expressing the exogenous NY-ESO-1 TCR also had mutated PDCD1 in the initial infusion product, but this percentage decreased to ~5% by 4 months post infusion, which is consistent with studies that have shown that PD-1- T cells are less capable of establishing memory. In this patient, NY-ESO-1-expressing cells increased the expression of IL7R and TCF7 over time, indicating central memory phenotype. This is in contrast with previous findings that showed that NY-ESO-1-expressing T cells, which had not been gene-edited, tended to develop a terminally differentiated phenotype and exhibited characteristics of exhaustion.
In this pilot study, two of the three treated patients had stable disease, which was the best clinical response. One patient had a significant (~50%) and sustained (for 4 months) decrease in an abdominal tumor mass, but had progression in other lesions. Ultimately, all three patients progressed; one of the patients died of progressive disease, and the other two are receiving other therapies. In the two patients with multiple myeloma, there was a decrease in the target antigens (NY-ESO-1 and/or LAGE-1), though residual tumor remained, possibly indicating an on-target effect that resulted in tumor editing. Blood samples obtained from patients three to nine months post infusion and expanded in culture in the presence of the NY-ESO-1 peptide demonstrated antigen-specific cytotoxicity in all three patients.
In this first-in-human clinical trial, Stadtmauer and Fraietta et al. demonstrated the initial safety and feasibility of using CRISPR-Cas9 to edit multiple genes in autologous T cells for the treatment of advanced, refractory cancer. Future studies with more patients will be necessary to assess the full safety profile and the efficacy of this approach.
by Anna Scherer




