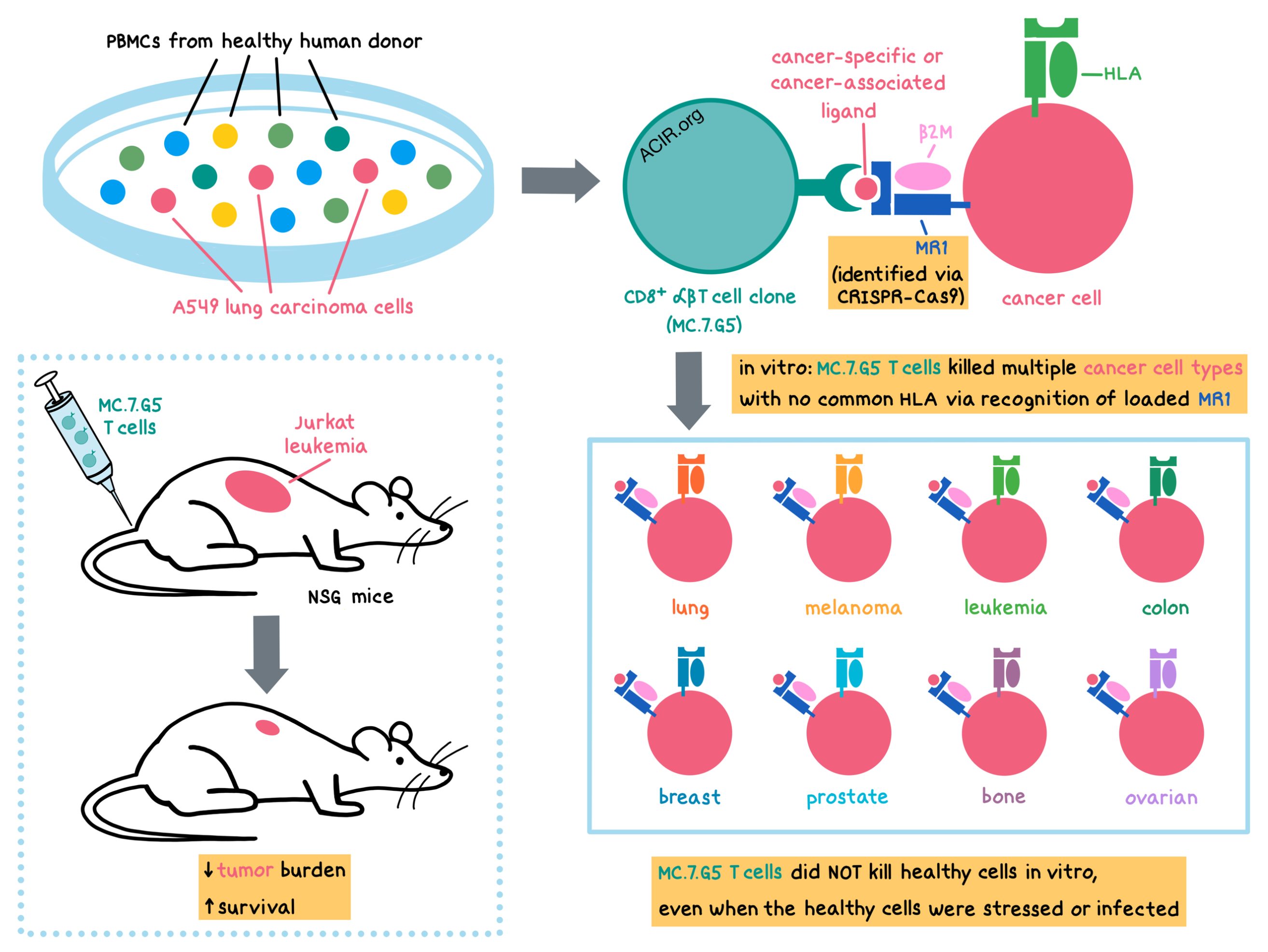
The holy grail of cancer research is finding one treatment that kills all types of cancer in every affected individual. With this lofty goal in mind, Crowther and Dolton et al. explored unconventional T cells and identified a T cell clone capable of killing many different types of cancer in an HLA-independent fashion while sparing healthy cells. The results were recently published in Nature Immunology.
The researchers began by collecting peripheral blood mononuclear cells (PBMCs) from a healthy donor and culturing them with A549 lung carcinoma cells (the HLAs of the donor and A549 cells were mismatched). From this culture, they isolated a CD8+ αβT cell clone (MC.7.G5), which had proliferated in vitro in response to tumor cells even in the presence of MHC-I- or MHC-II-blocking antibodies. Testing the effects of this clone on other cancers revealed that the MC.7.G5 clone killed multiple cancer cell lines (including lung, melanoma, leukemia, colon, breast, prostate, bone, and ovarian) that had no common HLA molecules, as well as primary cancer cells (ovarian and melanoma). Importantly, the MC.7.G5 clone did not kill a range of healthy cell types.
Given the cancer cell-specific, HLA-independent killing abilities of the MC.7.G5 clone, the researchers explored its mechanism of action. To accomplish this, Crowther and Dolton et al. utilized genome-wide CRISPR-Cas9 protein-coding screening and found that the MC.7.G5 T cell clone recognized cancer cells via the ubiquitously expressed, mostly monomorphic, MHC-I-related protein MR1, but not via the MHC-I or MHC-II molecules. The T cells recognized target cancer cells even at levels of MR1 expression that were below detection limits of surface staining for MR1.
To understand how the MC.7.G5 clone targeted the MR1 molecule, the researchers tested various known MR1 recognition mechanisms. MC.7.G5 T cells did not recognize a mutated version of MR1 that could be expressed on the cell surface without a bound ligand. In addition, recognition of target cells decreased when MR1 was loaded with either bacteria or vitamin B-related metabolite MR1 ligands. Thus, MC.7.G5 T cells did not recognize MR1 by itself, nor did they recognize the MR1 molecule loaded with known ligands. The researchers hypothesized that the MC.7.G5 clone recognized MR1 only when it was loaded with a ligand (possibly a metabolite) that was specific to, or associated with, cancer cells. The exact nature of the MR1-bound ligand that is recognized by the MC.7.G5 clone remains unknown at this time. Encouragingly, an MC.7.G5-like T cell clone was grown from a second donor, suggesting that MR1-restricted, cancer-specific T cells may exist in multiple individuals.
Despite recognition and killing of a diverse range of cancer cell lines or primary cancer cells, safety testing revealed that the MC.7.G5 clone did not kill healthy cells derived from various tissues, including smooth muscle, lung (fibroblast, alveolar, bronchus), skin (melanocytes), intestine, pancreas, kidney, and liver. Additionally, MC.7.G5 T cells were not activated by immature or mature monocyte-derived dendritic cells, mature Langerhans cells, resting or activated donor T and B cells, lymphoblastoid cells or normal renal epithelial cells undergoing cell stress, or healthy lung cells infected by bacteria. Therefore, the MC.7.G5 clone did not target a wide range of healthy cells, even under conditions of stress or infection.
To test the anticancer effects of the MC.7.G5 clone in vivo, Crowther and Dolton et al. inoculated immunodeficient NSG mice with Jurkat leukemia cells and then adoptively transferred MC.7.G5 T cells. Tumor burden was significantly reduced in mice receiving MC.7.G5 compared to mice with no MC.7.G5, as indicated by a significant decrease in leukemia cells in the bone marrow and spleen. MC.7.G5 recognition and killing of cancer cells in vivo was dependent on MR1 expression on the target cells. MC.7.G5 antitumor effects increased mouse survival, with the median survival of mice receiving the MC.7.G5 T cells being double the survival of mice without MC.7.G5 T cells (61 days versus 31 days, respectively).
Exploring the potential clinical relevance of targeting MR1 on cancer cells, the researchers isolated T cells from PBMCs of patients with stage IV melanoma and transduced them with the MC.7.G5 TCR. Transduced T cells recognized and killed autologous and nonautologous MR1+ melanoma cells, but not healthy cells (including melanocytes, pancreatic cells, and lung cells). Thus, transduction of MC.7.G5 TCR allowed the patients’ T cells to kill cancer cells in an HLA-independent manner.
Discovery of an HLA-independent TCR that recognizes the invariant MR1 molecule across many cancer types without targeting healthy cells provides a path toward the development of pan-cancer immunotherapeutic approaches, including TCR-engineered T cells and, once the ligand is identified, therapeutic anti-cancer vaccines.
by Anna Scherer
MEET THE RESEARCHER
This week, co-first author Michael Crowther answered our questions.

What prompted you to tackle this research question?
This study came about accidentally, while researching T cells that recognise bacteria. Luckily, I was based in a great T cell research group that encouraged me to focus on this surprise cancer-reactive T cell where we discovered its broad potential.
What was the most surprising finding of this study for you?
The most surprising finding of this study was that the MC.7.G5 T cell clone did not respond to healthy cells. We did not expect it to leave healthy cells untouched, even after stressing healthy cells harshly, due to its recognition of most cancer cells regardless of their source. This suggested it was truly cancer-specific, although more experiments need to be carried out to confirm this.
What was the coolest thing you’ve learned (about) recently outside of work?
I recently visited Australia to visit friends where I discovered the amazing wildlife and nature present there. It was a really great, life-changing experience and it showed me how important it is to protect these amazing places




