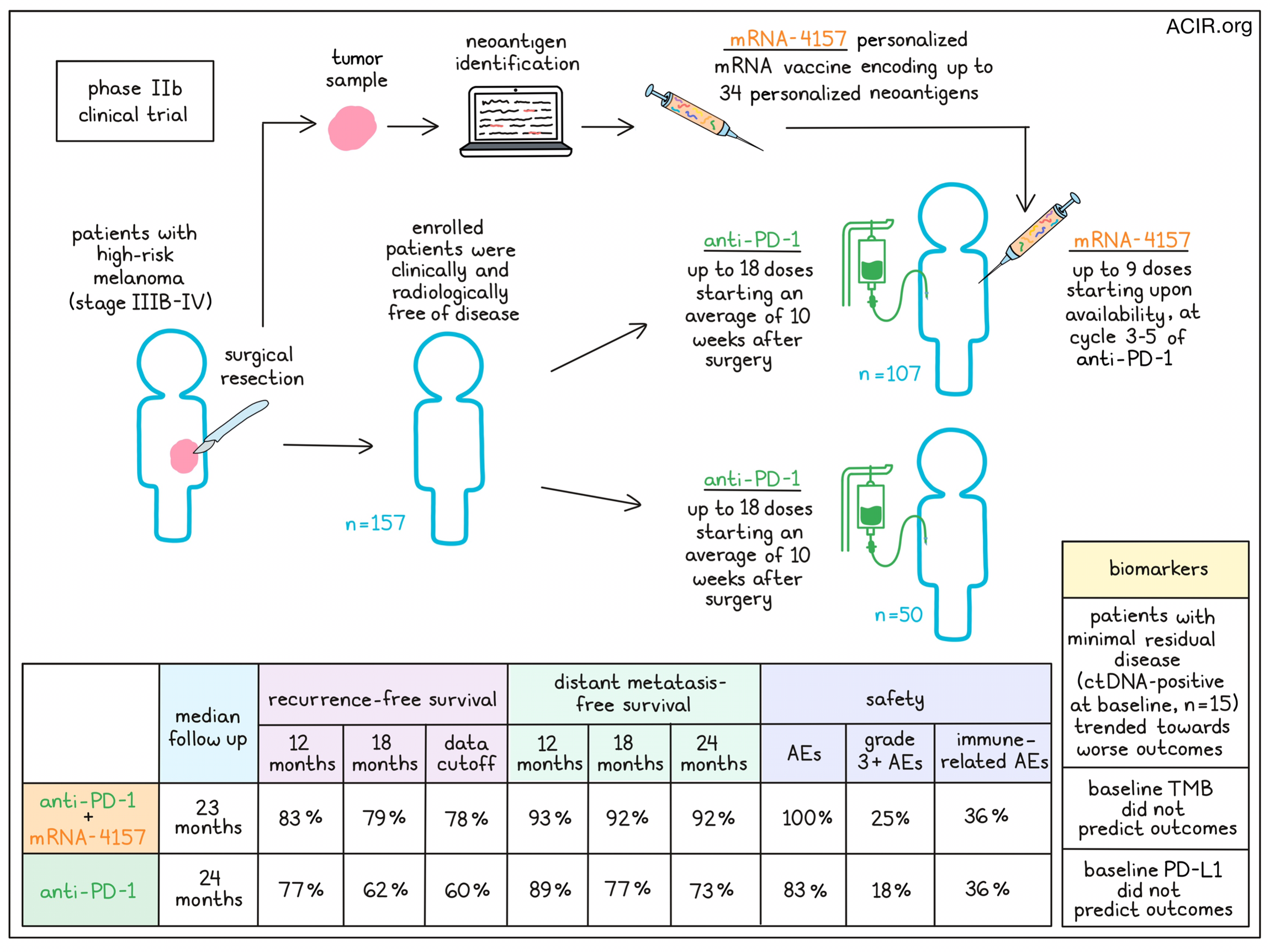
Following surgical resection of high-risk cutaneous melanoma, patients are generally treated with checkpoint inhibitors to prevent tumor recurrence, but a high proportion of patients still recur within 5 years. In an effort to reduce these recurrence rates, Weber et al. evaluated the addition of adjuvant personalized neoantigen vaccines (mRNA-4157) in a phase IIb clinical trial. The results were recently published in The Lancet.
Made using the same mRNA vaccine platform that is implemented for the SARS-CoV-2 (COVID-19) vaccine, RNA-4157 is an mRNA-based individualized neoantigen therapy. Based on samples of tumor and healthy tissue, researchers identify a patient’s HLA type and their tumor’s unique neoantigens, encode up to 34 neoantigens in mRNA, and encapsulate that mRNA in lipid nanoparticles. In preclinical work and a phase I study, mRNA-4157 was shown to induce robust antigen-specific T cell responses to encoded neoantigens, and to enhance antitumor responses in combination with pembrolizumab in the adjuvant setting.
In their recent phase IIb study, 157 adult patients with completely resected stage IIIB–IV melanoma, who were clinically and radiologically disease-free at the time of enrollment, were randomized to receive either mRNA-4157 plus pembrolizumab (n=107) or pembrolizumab monotherapy (n=50). Despite some holdups due to the production of COVID-19 vaccines, vaccine manufacturing was feasible, with only one patient for whom a vaccine could not be manufactured due to insufficient tissue. Patients in both the monotherapy and combination groups received 200mg of pembrolizumab intravenously every 3 weeks for up to 18 doses; the average time from surgery to the first dose of pembrolizumab was 10 weeks. For patients in the vaccine treatment group, 1mg of mRNA-4157 was given intramuscularly on the same days as the pembrolizumab for up to 9 doses, starting at the earliest availability of the vaccine, with 81% of patients beginning at pembrolizumab cycle three, 11% at cycle four, and 9% at cycle five. A majority of patients had protocol variations, largely due to disruptions related to the COVID-19 pandemic.
For this trial, the primary endpoint was recurrence-free survival, which was defined as the time from the first dose of pembrolizumab to the date of first recurrence (which could be local, regional, or a distant metastasis), a new primary melanoma, or death from any cause. At a median follow-up of 23 months for the combination group and 24 months for the monotherapy group, recurrence-free survival was longer in the combination group. Looking at recurrence-free survival over time, the 12-month recurrence-free survival rates were 83% in the combination group and 77% in the monotherapy group, and the respective 18-month recurrence-free survival rates were 79% and 62%. At the time of data cutoff, the recurrence free-survival rates were 78% and 60%.
Since most first recurrences are distant metastases, a secondary endpoint of this trial was distant metastasis-free survival, which like recurrence-free survival, was longer in the combination group compared to the monotherapy group. At 12 months, the distant metastasis-free survival rates were 93% and 89%, respectively. Over time, that gap widened, with 18-month distant metastasis-free survival rates of 92% and 77%, and 24-month distant metastasis-free survival rates of 92% and 73%. Further, a higher proportion of patients in the monotherapy group had a distant recurrence event as their recurrence-free survival event compared with the combination group. These results suggest that patients treated with mRNA-4157 had a reduced or delayed risk of disease recurrence at distant sites, and that mRNA-4157 likely improves outcomes by mediating protection against distant metastases over time.
Another secondary endpoint of this trial was safety and tolerability. Among all patients included in the safety analysis (n=154), most treatment-related adverse events were grade 1–2, with 100% of patients in the combination group and 83% of patients in the monotherapy group reporting adverse events of some kind. The most common adverse events related to the mRNA-4157 were fatigue, chills, and injection site pain/reactions. Grade 3 or higher treatment-related adverse events occurred in 25% of patients in the combination group (with 12% related to the mRNA-4157) versus 18% of patients in the monotherapy group. No grade 4 or 5 events related to the mRNA-4157 occurred, and the immune-mediated adverse event frequency was 36% in both groups. Adverse events related to pembrolizumab were consistent with its use as a monotherapy. These results indicate a higher rate of mostly low-grade adverse events with the addition of a second agent, but treatment was considered both safe and tolerable. Further, while the most common reason to discontinue treatment in the combination treatment was adverse events, the most common reason in the monotherapy group was tumor recurrence.
Additional exploratory endpoints evaluated in this study included assessing circulating tumor DNA (ctDNA), tumor mutation burden (TMB), and PD-L1 status in baseline tumors as potential predictive biomarkers. In patients who were ctDNA-positive at baseline (minimal residual disease; n=15) versus ctDNA-negative (n=110), the researchers noted a trend towards shorter recurrence-free and distant metastasis-free survival, regardless of their treatment. TMB-high and TMB-low subgroups had a similar recurrence-free survival benefit in the combination group versus the monotherapy group, as did PD-L1-positive and PD-L1-negative subgroups, suggesting that these features are not predictive of outcomes in the combination group versus the monotherapy group for melanoma.
Overall, the results of this trial show that the addition of adjuvant mRNA-4157 to pembrolizumab in patients with resected high-risk melanoma prolonged recurrence-free survival and showed a manageable safety profile. In particular, mRNA-4157 appeared to reduce the risk of distant metastases over a prolonged period of time. An extended follow-up of this study is currently ongoing to establish the durability of the treatment effect. The combination of mRNA-4157 and pembrolizumab is also being evaluated in a phase III clinical trial.
Write-up and image by Lauren Hitchings




