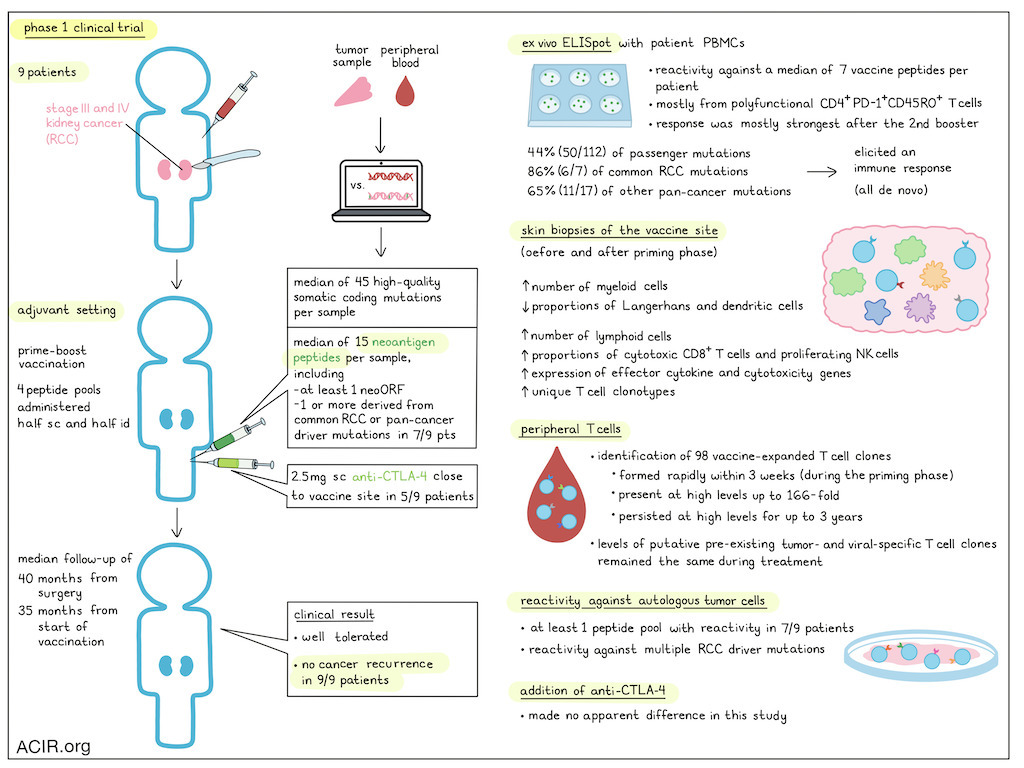
Kidney cancer is a common cancer type, and about 75-80% of cases are clear cell renal cell carcinoma (RCC). Patients with RCC are often faced with a high risk of cancer recurrence after complete surgical removal. In a recent Phase I clinical trial published in Nature, Braun and colleagues investigated the potential of a personalized cancer vaccine for patients with RCC following tumor resection. The innovative approach of this vaccine lies in its ability to stimulate a targeted immune response against specific mutations in each patient’s tumor (neoantigens), a strategy that has gained traction in recent years.
A significant challenge in developing neoantigen-targeted vaccines for RCC is the characteristically low mutational burden of RCC tumors. Despite this, the researchers were able to successfully produce and administer vaccines to nine patients with advanced RCC (seven with stage III and two with metastatic stage IV disease). Tumor analysis identified a median of 45 high-quality somatic coding mutations per sample, and utilized predictive algorithms to select neoantigens for inclusion in the vaccine product based on their likelihood to induce an immune response. A median of 15 neoantigen-containing peptides were synthesized for each patient, including at least one peptide from a novel open reading frame caused by a frameshift insertion or deletion. Additionally, for seven of the nine patients, one or more neoantigen peptides derived from common RCC or other pan-cancer driver mutations were incorporated.
Braun and his team combined the peptides into four pools of up to 5 peptides each. The peptide pools were mixed with the adjuvant poly-ICLC and administered (half subcutaneously, half intradermally) at separate, non-rotating limbs (i.e., each pool into the same limb) on days 1, 4, 8, 15, and 22 (priming phase) and then on weeks 12 and 20 (booster phase). Five patients were also given 2.5 mg of the anti-CTLA-4 antibody ipilimumab subcutaneously within 1 cm of the vaccine site.
The vaccine was well tolerated. While all patients experienced minor reactions at the injection site and most patients (8/9) had temporary flu-like symptoms, no grade 3 or higher side effects were observed. Importantly, during the follow-up period (median of 40.2 months from surgery and 34.7 months from the start of vaccination), none of the nine patients experienced cancer recurrence, suggesting the potential efficacy of the vaccine in preventing disease relapse.
The vaccine's mechanism of action relies on triggering a robust and durable immune response against the patient's tumor-specific neoantigens. In this trial, the vaccine successfully triggered an ex vivo ELISpot response in PBMCs of all of the patients, and this response was strongest after the second booster at week 24 for most patients (6/9). Detailed deconvolution after in vitro stimulation revealed reactivity against a median of 7 peptides per patient, predominantly from polyfunctional, antigen-experienced, memory phenotype PD-1+CD45RO+ CD4+ T cells. Across all patient samples, the immunogenicity of the neoantigen peptides was notable, with 44% (50/112) of passenger mutations, 86% (6/7) of common RCC driver mutations, and 65% (11/17) of pan-cancer driver mutations eliciting an immune response. No reactivity was detected to any of the peptides in PBMC samples prior to vaccination.
To further understand the immune response at the vaccine site, the researchers performed scRNAseq and TCRseq analyses on skin biopsies taken near to the site of vaccination before and 2-3 days after the first five vaccine doses in the priming phase. These analyses revealed an increase in the absolute number of infiltrating myeloid and lymphoid cell populations, but a relative decrease in the proportion of Langerhans cells and dendritic cells following vaccination, potentially due to enhanced migration of these APCs to the draining lymph nodes. Additionally, there was a trend towards higher proportions of cytotoxic CD8+ T cells and proliferating NK cells with increased expression of effector cytokine and cytotoxicity genes. A significant increase in unique T cell clonotypes was also observed.
The researchers also tracked individual T cell clonotypes in peripheral blood samples collected before, during, and after vaccination using bulk TCRβ RNA sequencing. They focused on circulating T cell clones that showed an at least ten-fold increase in response to vaccination; only clonotypes that were also identified using RNAseq and TCRseq data from skin biopsies obtained from delayed-type hypersensitivity testing using the combined peptide pools as immunogen were considered vaccine-specific. The team identified 98 vaccine-expanded T cell clones, with a mean expansion of 166-fold. These new T cell clonotypes were observed as early as within 3 weeks following vaccination (during the vaccine priming phase). The majority of these T cell clones were from the CD4 compartment, and could be detected at high levels for up to 3 years. Notably, the levels of pre-existing putative tumor-specific and viral-specific T cell clones (identified based on gene signatures found in single-cell sequencing data in the baseline resected RCC tumors), stayed about the same throughout vaccination.
Finally, the researchers checked whether vaccine-induced T cells could recognize and react against each patient’s own tumor cells. They collected peripheral T cells after the first booster and expanded them against individual vaccine peptides derived from RCC driver mutations or peptide pools. Overall, 7 of the 9 trial participants had at least one peptide pool with reactivity against autologous tumor cells, and reactivity to multiple individual RCC driver mutations was observed, further supporting the vaccine's potential to induce a targeted antitumor response.
Ipilimumab was co-delivered near to the vaccine in 5 of the 9 patients to potentially enhance T cell priming while reducing systemic toxicities. Subcutaneous ipilimumab was well tolerated. Interestingly, the researchers did not observe any immunological differences between patients who received ipilimumab and those who did not, which may indicate an absence of an effect on priming, or could be a confounding consequence of the dose or the analytical procedures used.
Overall, this Phase I clinical trial demonstrated the feasibility and potential of a personalized neoantigen cancer vaccine for patients with RCC following surgical tumor removal. The vaccine was well tolerated and elicited durable immune responses, with an encouraging absence of disease recurrence among this small set of patients, offering a potential solution for patients with RCC who are at high risk of cancer recurrence after surgery. While further research is needed to confirm these findings in larger trials, this study represents a promising step towards adjuvant personalized cancer immunotherapy, even in tumors with low mutational burden.
Write-up and image by Ute Burkhardt
Meet the researcher
This week, David A. Braun, Derin Keskin and Patrick A. Ott answered our questions.

What was the most surprising finding of this study for you?
We were very pleasantly surprised by the magnitude, durability, and antitumor specificity of the immune response following vaccination. While some of the major aim of this phase I study was to investigate the feasibility and safety of this approach, it was tremendously encouraging to see that all 9 patients treated with the vaccine had ex vivo immune responses against neoantigens, and that these vaccine-reactive T cells persisted for months or years after vaccination was complete. Because we had access to serial PBMC samples and viably preserved tumor from each patient, we were able to test post-vaccination T cells for direct antitumor reactivity. Remarkably, in 7 of 9 patients, vaccination led to T cell responses against autologous tumors. We find it very promising that none of the 9 patients treated with the personalized cancer vaccine had a recurrence of their kidney cancer during the study, though we interpret these results with substantial caution, given the limited number of patients.
Surprisingly, one of the biggest challenges during the inception phase of the study was not “biological feasibility” (i.e. are we able to find enough mutations in kidney cancer?, can we manufacture the individual peptides for the vaccine? Etc,), but rather, navigating through many regulatory hurdles and aligning resulting delays with the timeline expectations of the financial backers of the study. The study is a true testament of “academic grit”, as it took as 9 years to complete a 9-patient study.
What is the outlook?
We believe the outlook is very promising. Our initial study indicates that the vaccine is doing exactly what we would hope for – leading to durable, antitumor immunity. Further, there was an encouraging signal of clinical activity. However, the clinical effectiveness of this approach now needs to be studied in a systematic and rigorous fashion, incorporating the current standard-of-care therapy (PD-1 blockade). We are now actively investigating the clinical efficacy of neoantigen-targeting personalized cancer vaccines through a larger, randomized phase II trial – INTerpath-004.
Who or what has been a major source of inspiration or motivation for you throughout your career?
DAB: I have been tremendously fortunate to have many scientific role models and mentors throughout my training and my career. I have also been exceptionally lucky to have the support of my family, and specifically my wife and daughters. Further, I am pushed and motivated each day by the courageous patients and caregivers I see in the clinic, and by the brilliant and motivated trainees in my laboratory. Finally, I look to my own mother, whose tremendous grace, selflessness, and kindness while battling her own cancer continues to serve as an inspiration for me.
DK: Witnessing the hardships and burdens of cancer patients has been an inspiration throughout my career. My urgent motivation for immuno-oncology research is also self-preservation after supporting close relatives with cancer. Neoantigen-based cancer vaccines promise a very safe and low side-effect alternative to classical cytotoxic cancer chemotherapy.
What was the coolest thing you’ve learned (about) recently outside of work?
PAO: My recent trip to Peru reminded me of the Nazca Lines, an archeological site in the South of the country, where a vast area shows marks in the desert sand that seem random on the ground, but assemble to mysterious figures when viewed from high up in the air. I was intrigued to learn that a team of archeologists using drones and AI was recently able to double the number of these lines, which previously took almost a century to unravel.




