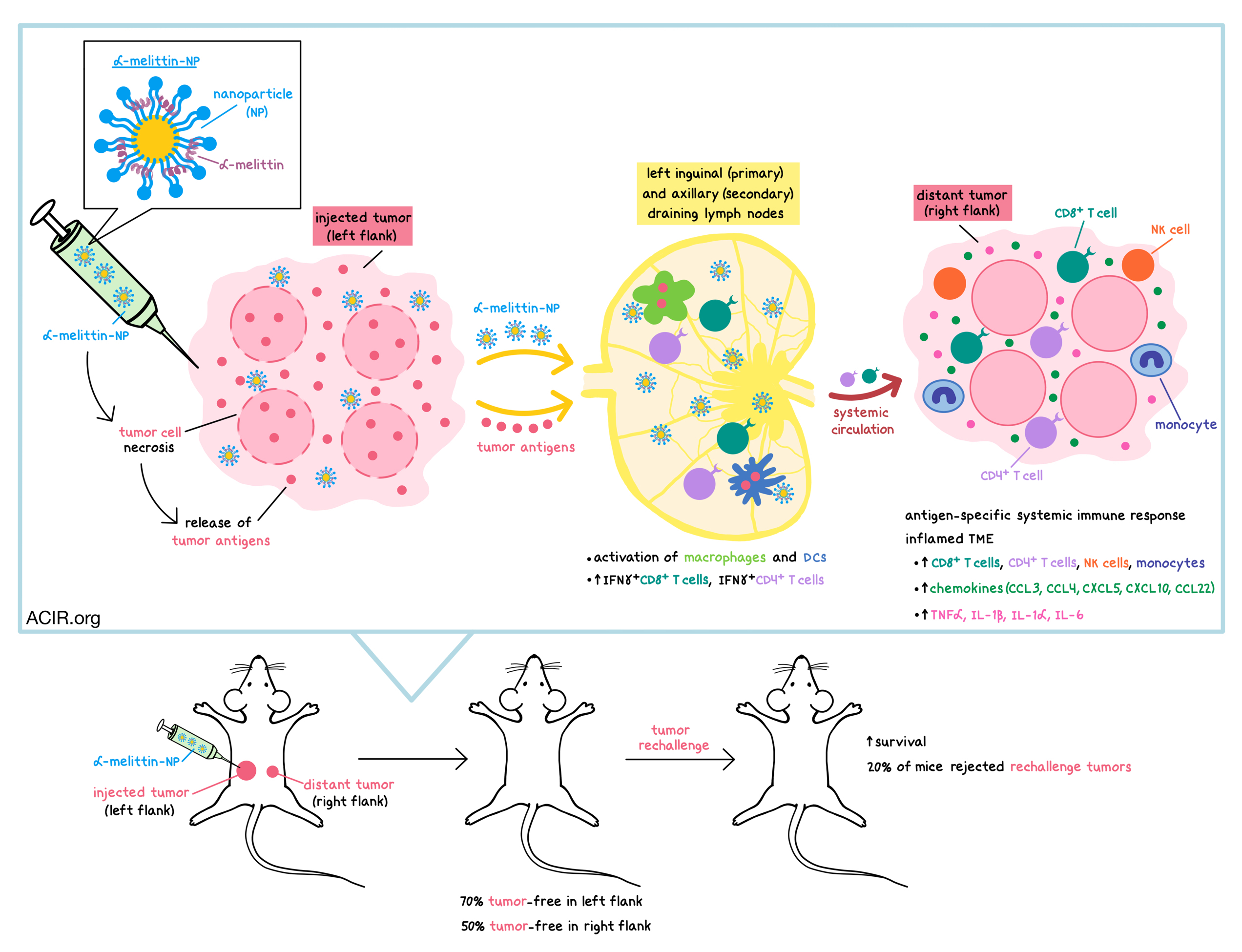
Targeting the tumor where it lives and turning the tumor against itself was what Yu and Dai et al. had in mind when they developed a one-two punch nanovaccine comprising a lipid nanoparticle (NP) loaded with melittin (a peptide found in European bee venom), called α-melittin-NP. The vaccine, which contained no tumor antigens, promoted tumor cell lysis, releasing whole-cell tumor antigens, and targeted to the lymph nodes, where it stimulated antigen-presenting cells and elicited a systemic antitumor response. The results were recently published in Nature Communications.
Melittin is a small (~2500 dalton) host defense peptide in bee venom that stimulates various aspects of immunity. By itself, melittin has only a narrow safe dose range, with its major side effect being rupture of red blood cells (hemolysis). To overcome this toxicity and improve delivery to lymph nodes, Yu and Dai et al. incorporated melittin in 10-20 nm nanoparticles composed of phospholipids. Imaging data showed that after a subcutaneous injection in mice, α-melittin-NPs and α-peptide-NPs (containing a control peptide) accumulated in inguinal and axillary draining lymph nodes. As expected, free melittin did not target to the lymph nodes, and instead was directly absorbed into the blood, where it led to hemolysis. Within the lymph nodes, α-melittin-NPs and α-peptide-NPs were mostly taken up by macrophages and dendritic cells (DCs), but very few of them ended up in B cells or T cells. Moreover, α-melittin-NPs increased the percentage of activated macrophages and DCs, while free melittin and α-peptide-NPs did not.
Exploring the safety of α-melittin-NPs, the researchers incubated α-melittin-NPs or free melittin with bone marrow-derived DCs (BMDCs), bone marrow-derived macrophages (BMDMs), or B16F10 melanoma cells. They observed that free melittin was cytotoxic to all three types of cells, however, α-melittin-NPs killed the tumor cells while sparing the BMDCs and BMDMs at a certain concentration range. The researchers hypothesized that there may be two reasons for the differential cytotoxicity to these cell populations. First, cancer cells have a slightly higher negative charge in their membranes than normal cells, and therefore may be more likely to attract α-melittin-NPs. Second, Yu and Dai et al. observed that α-melittin-NPs were endocytosed by BMDMs and BMDCs (where they localized in intracellular membranes), but were distributed in the cell membrane of the tumor cells, making the tumor cells more susceptible to cell membrane disruption and necrosis. Using mice inoculated with fluorescently labeled B16F10 tumor cells, the researchers confirmed that both free melittin and α-melittin-NPs made the tumor cell membrane more permeable and induced the release of tumor antigens in vivo.
Next, the researchers turned to mouse models to assess the antitumor effect of α-melittin-NPs in vivo. In a bilateral flank B16F10 tumor model (where the tumor in the right flank was implanted 4 days after the tumor in the left flank), intratumoral injection of α-melittin-NPs into the left flank drastically suppressed tumor growth in both the injected tumor (by 95%) and the distant, non-injected tumor (by 92%) compared with control (PBS injection) at 20 days post left tumor inoculation. A smaller inhibitory effect on tumor growth was observed with free melittin (37% in the injected tumor and 66% in the distant tumor). At 60 days post tumor inoculation, among the mice treated with α-melittin-NPs, 70% were tumor-free in the left flank and 50% were tumor-free in the right flank. In contrast, among mice treated with free melittin, 10% were tumor-free in the left flank and none were tumor-free in the right flank. Upon tumor rechallenge, mice treated with α-melittin-NPs had prolonged survival, and 20% of mice treated with α-melittin-NPs completely rejected the tumors.
Examining the antitumor response in more detail, the researchers observed that α-melittin-NPs were mainly found in the injected tumor site, and were practically undetectable in the contralateral tumor, suggesting that the reduced growth of the distant tumor was due to a systemic immune response, and not due to direct tumor cell killing by α-melittin-NPs. Lymphocytes collected from tumor-draining lymph nodes and restimulated with B16F10 tumor lysate-pulsed DCs showed that α-melittin-NPs increased the frequencies of IFNγ+CD8+ T cells (12-fold) and IFNγ+CD4+ T cells (7-fold) 21 days after tumor inoculation. In contrast, free melittin only moderately increased the frequency of IFNγ+CD8+ T cells (3-fold). Analysis of serum collected at day 21 and incubated with B16F10 tumor cells showed that there was no difference in the increase of IgG+ cells between free melittin and α-melittin-NP treatment groups, indicating that the enhanced antitumor effect observed with α-melittin-NPs was mainly due to cellular immunity and not an antibody response. Furthermore, by inoculating mice with two different types of tumors (E0771 breast cancer cells in the left flank, B16F10 cells in the right flank), Yu and Dai et al. showed that the α-melittin-NP-induced systemic antitumor response was antigen-specific, as the treatment failed to inhibit growth of the distant tumor when the injected and distant tumor types were mismatched.
A more in-depth analysis of leukocytes infiltrating the distant tumor in mice with matching tumors in both flanks revealed that α-melittin-NPs significantly altered the microenvironment of the distant tumor. 14 days after treatment of the left tumor, α-melittin-NPs increased the number of CD8+ T cells, CD4+ T cells, NK cells, and monocytes. Free melittin increased the number of CD4+ T cells and monocytes, but not CD8+ T cells, compared with PBS treatment. The α-melittin-NP-induced intratumoral leukocyte infiltration was driven by increased levels of chemokines (CCL3, CCL4, CXCL5, CXCL10, CCL22) that play a role in the recruitment of T cells, NK cells, and monocytes. The treatment also increased levels of proinflammatory cytokines, including TNFα, IL-1β, IL-1α, and IL-6. These data suggest that α-melittin-NPs led to an inflamed tumor microenvironment in the non-injected tumor.
The results of this study suggest that α-melittin-NPs could serve as an effective in situ nanovaccine without the need for antigen loading for cancer types that are accessible for intratumoral injection, providing the benefits of inducing a broad, systemic immune response, delaying tumor growth, and mediating some complete regressions of uninjected tumors, while requiring a relatively simple preparation and exerting no apparent side effects.
by Anna Scherer




